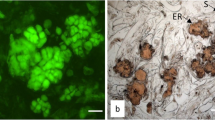
Abstract
A cryopreservation method developed earlier was modified for freezing of calli derived from mature embryos of four spring wheat (Triticum aestivum L.) pure lines. The effects of particular stages of cryopreservation protocol on water content, number of calli recovering growth, and rate of morphogenesis were analyzed. Regrowth was observed in 90.5–100% of calli after dehydration and in 93.3–100% after freezing-thawing. Dehydration, but not freeze-thawing significantly decreased the frequency of morphogenetic variation.
Similar content being viewed by others
References
Bajaj, Y.P.S., Freeze-Preservation of Plant Cells—a Novel Approach to the Conservation of Gemplasm, in Genetics and Wheat Improvement, Gupta, A.K., Ed., Delhi: Oxford, IBH, 1980, pp. 141–149.
Bajaj, Y.P.S., Survival of Somatic Hybrid Protoplasts of Wheat × Pea and Rice × Pea Subjected To −196°C, Indian J. Exp. Biol., 1983, vol. 21, pp. 120–122.
Blakesley, D., Mazrooei, S.A., Bhatti, M.H., and Henshaw, G.G., Cryopreservation of Non-Encapsulated Embryogenic Tissue of Sweet Potato (Ipomoea batatas), Plant Cell Rep., 1996, vol. 15, pp. 873–876.
Butenko, R.G., Popov, A.S., Volkova, L.A., et al., Recovery of Cell Cultures and Their Biosynthetic Capacity after Storage of Dioscorea deltoidea and Panax ginseng Cells in Liquid Nitrogen, Plant Sci. Lett., 1984, vol. 33, pp. 285–292.
Butenko, R.G., Popov, A.S., Volkova, L.A., et al., Plant Cell Viability at Different Deep Freezing Protocols, Tsitologiya, 1983, no. 10, pp. 1191–1196.
Chaturvedi, H.C. and Mitra, G.C., A Shift in Morphogenetic Pattern in Citrus Callus Tissue during Prolonged Culture, Ann. Botany, 1975, vol. 39, pp. 683–687.
Chen, T.H.H., Kartha, K.K., and Gusta, L.V., Cryopreservation of Wheat Suspension Culture and Regenerable Callus, Plant Cell Tiss. Organ Cult., 1985, vol. 4, pp. 101–109.
Cho, J.S., Hong, S.M., Joo, S.Y., et al., Cryopreservation of Transgenic Rice Suspension Cells Producing Recombinant HCTLA4Ig, Applied Microbiol. Biotechnol., 2007, vol. 73, no. 6, pp. 1470–1476.
Danso, K.E. and Ford-Lloyd, B.V., Cryopreservation of Embryogenic Calli of Cassava using Sucrose Cryoprotection and Air Desiccation, Plant Cell Rep., 2004, vol. 22, pp. 623–631.
Dumet, D., Engelmann, F., Chabrillange, N., and Duval, Y., Cryopreservation of Oil Palm (Elaeis guineensis Jacq.) Somatic Embryos Involving a Desiccation Step, Plant Cell Rep., 1993, vol. 12, pp. 352–355.
Engelmann, F., Plant Cryopreservation: Progress and Prospects, In Vitro Cell. Dev. Biol. Plant., 2004, vol. 40, no. 5, pp. 427–433.
Karp, A., Somaclonal Variation As a Tool for Crop Improvement, Euphytica, 1995, vol. 85, pp. 295–302.
Keefe, P. and Henshaw, G., A Note on the Multiple Role of Artificial Nucleation of the Suspending Medium during Two-Step Cryopreservation Procedures, Cryoletters, 1984, vol. 5, pp. 71–78.
Kendall, E.J., Qureshi, J.A., Kartha, K.K., et al., Regeneration of Freezing-Tolerant Spring Wheat (Triticum aestivum, L.) Plants from Cryoselected Callus, Plant Physiol., 1990, vol. 94, pp. 1756–1762.
Linch, P.T., Benson, E.E., Jones, J., et al., The Embryogenic Potential of Rice Cell Suspensions Affects Their Recovery Following Cryogenic Storage, Euphytica, 1995, vol. 85, pp. 347–349.
Matsubayashi, Y. and Sakagami, Y., Phytosulfokine, Sulfated Peptides That Induce the Proliferation of Single Mesophyll Cells of Asparagus officinalis, L., Proc. Natl. Acad. Sci. USA. Cell Biology, 1996, vol. 93, pp. 7623–7627.
Mazur, P., Cryobiology: The Freezing of Biological System, Science, 1970, vol. 168, no. 3934, pp. 939–949.
Moukadiri, O., Lopes, C.R., and Cornejo, M.J., Physiological and Genomic Variations in Rice Cells Recovered from Direct Immersion and Storage in Liquid Nitrogen, Physiol. Plant., 1999, vol. 105, pp. 442–449.
Murashige, T. and Skoog, F., A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures, Physiol. Plant., 1962, vol. 15, no. 4, pp. 473–497.
Panis, B. and Lambardi, M., Status of Cryopreservation Technologies in Crops and Forest, in The Role of Biotechnology, Villa Gualino, Turin, Italy, March 5–7, 2005, pp. 43–54.
Plokhinskii, N.A., Matematicheskie Metody v Biologii (Mathematical Methods in Biology), Moscow: Mosk. Gos. Univ., 1978.
Popov, A.S. and Fedorovskii, D.N., Lesions of Plasmalemma of Diascorea Cells Cultured in vitro during Their Cryopreservation, Fiziol. Rast., 1992, vol. 39, no. 2, pp. 335–343.
Popov, A.S., Chernyak, N.D., and Paukov, V.N., Improvement of Reculturing of Cells after Their Storage in Liquid Nitrogen, Fiziol. Rast., 1984, vol. 31, no. 3, pp. 602–605.
Popov, A.S., Some Mechanisms of Plant Cell Cryodamage in vitro and Characteristic of Their Cryopreservation, Fiziol. Rast., 1993, vol. 40, no. 3, pp. 485–496.
Popova, E.V., Lee, E.J., Wu, C.H., et al., A Simple Method for Cryopreservation of Ginkgo Biloba Callus, Plant Cell Tiss. Organ Cult., 2009, vol. 97, pp. 337–343.
Pritchard, H.W., Grout, B.W.W., and Short, K.C., Osmotic Stress as a Pregrowth Procedure for Cryopreservation 1. Growth and Ultrastructure of Sycamore and Soybean Cell Suspensions, Ann. Botany, 1986, vol. 57, pp. 41–48.
Vysotskaya, O.N., Danilova, S.A., and Popov, A.S., Method of Cryopreservation in vitro of Meristems Isolated from Strawberry, RF Patent No. 2 302 107, Byull. Izobret., no. 19, 2007.
Salcheva, G. and Samygin, G.A., Microscopic observations of Freezing of Winter Wheat Tissues, Fiziol. Rast., 1963, vol. 10, no. 1, pp. 65–72.
Samygin, G.A., Prichiny vymerzaniya rastenii (Causes of Plant Winterkilling), Moscow: Nauka, 1974.
Samygin, G.A., Causes of Damage of Plant Cells by Extracellular Ice, Fiziol. Rast., 1994, vol. 41, pp. 614–625.
Sowa, S., Oleszczuk, S., and Zimny, J., A Simple and Efficient Method for Cryopreservation of Embryogenic Triticale Calli, ACTA Physiol. Plant, 2005, vol. 27, no. 2, pp. 237–243.
Steponkus, P., Dowgert, M., and Gordon-Kamm, W., Destabilization of the Plasma Membrane of Isolated Plant Protoplasts during a Freeze-Thaw Cycle: The Influence of Cold Acclimation, Cryobiology, 1983, vol. 20, no. 4, pp. 448–465.
Tantau, H. and Darffling, K., In vitro-Selection of Hydroxyproline-Resistant Cell Lines of Wheat (Triticum aestivum): Accumulation of Proline, Decrease in Osmotic Potential, and Increase in Frost Tolerance, Physiol. Plant., 1991, vol. 82, no. 2, pp. 243–248.
Trunova, T.I., Sugars as a Factor Increases Frost Hardiness of Plants, Izv. Akad. Nauk SSSR, Ser. Biol., 1972, no. 2, pp. 185–189.
Tumanov, I.I., Krasavtsev, O.A., and Trunova, T.I., Survival of Winter Wheat at −196°C as a Result of Vitrification, Dokl. Akad. Nauk SSSR, 1965, vol. 161, pp. 978–981.
Vannini, G.L. and Poli, F., Binucleation and Abnormal Chromosome Distribution in Euglena gracilis Cells Treated with Dimethyl Sulfoxide, Protoplasma, 1983, vol. 114, pp. 62–66.
Wang, W.C., Shang, X.M., Ycel, M., and Nguyen, H.T., Selection of Cultured Wheat Cells for Tolerance to High Temperature Stress, Crop Sci. Soc. Amer., 1993, vol. 33, pp. 315–320.
Wang, X.J., Loh, C.S., Yeoh, H.H., and Sun, W.Q., Differential Mechanisms to Induce Dehydration Tolerance by Abscisic Acid and Sucrose in Spathoglottis plicata (Orchidaceae) Protocorms, Plant Cell Environ., 2003, vol. 26, pp. 737–744.
Zhang, L., Rybczynski, J.J., Langenberg, W.G., et al., An Efficient Wheat Transformation Procedure: Transformed Calli with Long-Term Morphogenic Potential for Plant Regeneration, Plant Cell Rep., 2000, vol. 19, pp. 241–250.
Zhang, Y.X., Wang, J.H., Bian, H.W., and Zhu, M.Y., Pregrowth-Desiccation: A Simple and Efficient Procedure for the Cryopreservation of Rice (Oryza sativa (L.) Embryogenic Suspension Cells, Cryoletters, 2001, vol. 22, no. 4, pp. 221–228.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.I. Solov’eva, O.N. Vysotskaya, A.S. Popov, Yu.I. Dolgikh, 2010, published in Izvestiya Akademii Nauk, Seriya Biologicheskaya, 2010, No. 5, pp. 574–580.
Rights and permissions
About this article
Cite this article
Solov’eva, A.I., Vysotskaya, O.N., Popov, A.S. et al. Freezing of dehydrated calli of spring wheat (Triticum aestivum L.) in liquid nitrogen and their morphogenetic potential. Biol Bull Russ Acad Sci 37, 489–495 (2010). https://doi.org/10.1134/S1062359010050080
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359010050080