Abstract
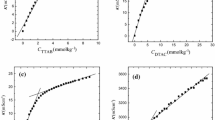
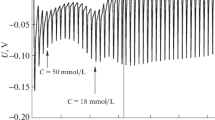
The effect of cosolvent on micellization of hexadecyltrimethyl ammonium bromide (CTAB) in aqueous solutions was studied. The conductivity of a mixture (cosolvent + water) as function of CTAB concentration was measured at different temperatures. Ethylene glycol and ethanol were used as a cosolvent. The conductivity data were used to determine the critical micelle concentration (CMC) and the effective degree of counterion dissociation of micelle in the temperature range 303.2 to 313.2 K. In all the cases studied, a linear relationship between log([CMC]mix/mol dm−3) and the mass fraction of cosolvent in solvent mixture has been observed. The free energy (ΔG 0mic ), enthalpy (ΔH 0mic ), and entropy (ΔS 0mic ) of micellization were determined using the temperature dependence of CMC. The dependence of these thermodynamic parameters on solvent composition was determined. The standard free energy of micellization was found to be negative in all cases and becomes less negative as the cosolvent content increases. The enthalpy and entropy of micellization are independent of temperature in pure water, while ΔH 0mic and ΔS 0mic decrease dramatically with temperature in mixed cosolvents. Furthermore, the entropic contribution is larger than the enthalpic one in pure water, while in the mixed solvents, the enthalpic contribution predominates.
Similar content being viewed by others
References
De Lisi, R. and S. Milato, Chem. Soc. Rev., 1994, vol. 23, p. 67.
Alizadeh, N., Gharibi, H., and Shamsipur, M., Bull. Chem. Soc. Jpn., 1995, vol. 68, p. 730.
Binana-Limbele, B. and Zana, R., Colloid Polymer Sci., 1989, vol. 267, p. 440.
Sharma, V.K., Yadav, O.P., and Singh, J., Colloids Surf., 1996, vol. 110, p. 23.
Zana, R., Adv. Colloid Interface Sci., 1996, vol. 57, p. 1.
Bakshi, M.S., Crisantino, R., De Lisi, R., and Milato, S., J. Am. Chem. Soc., 1993, vol. 97, p. 6914.
Myers, D., Surfactant Science and Technology, Weinheim: VCH Publishers, 1988.
Wasserman, A.M., Russ. Chem. Soc. Rev., 1994, vol. 231, p. 373.
Sjöberg, M., Henrikson, U., and Warnheim, T., Langmuir, 1990, vol. 6, p. 1205.
Beesley, A.H., Evans, D.F., and Laughlin, R.G., J. Phys. Chem., 1988, vol. 92, p. 791.
Chauhan, M.S., Kumar, G., Kumar, A., Sharma, K., and Chauhan, S., Colloids Surf., 2000, vol. 180, p. 111.
Almgren, M., Swarup, S., and Löfroth, J.E., J. Phys. Chem., 1985, vol. 89, p. 4621.
Gracie, K., Turner, D., and Palepu, R., Can. J. Chem., 1996, vol. 74, p. 1616.
Rosen, M., Surfactants and Interfacial Phenomena, New York: Wiley; 1989.
Zana, R., Cationic Surfactants, vol. 37, Rubingh, D.N. and Holland, P.M., Eds., New York: Marcel Dekker, 1991.
Shamsipur, M., Alizadeh, N., and Gharibi, H., Bull. Chem. Soc. Jpn., 1996, vol. 69, p. 311.
Akbaş, H. and Kartal, Ç., Spectrochim. Acta A, 2005, vol. 61, p. 961.
Kartal, Ç. and Akbaş, H., Dyes Pigments, 2005, vol. 65, p. 191.
Del Castillo, J.L., Suarez-Filloy, M.J., Castedo, A., Svitova, T., and Rodriguez, J.R., J. Phys. Chem. B, 1997, vol. 101, p. 2782.
Dominoques, A., Fermandez, A., Gonzalez, N., Inglesias, E., and Montonegro, L., J. Chem. Educ., 1997, vol. 74, p. 1227.
Lianos, P. and Lang, J., J. Colloid Interface Sci., 1983, vol. 1, p. 222.
Nagarajan, R. and Wang, C.C., Langmuir, 2002, vol. 16, p. 5242.
Ylhautala, M., Vaara, J., Ingman, P., Jokisaari, J., and Diehl, P., J. Phys. Chem. B, 1997, vol. 100, p. 32.
CRC Handbook of Chemistry and Physics, 75th edition, 6-(156.158), Boca Raton: CRC Press, 1995.
Ray, A., Nature, 1971, vol. 231, p. 313.
Sjoberg, M., Hendrikson, U., and Warnheim, T., Langmuir, 1990, vol. 6, p. 1205.
Palepu, R., Gharibi, H., Bloor, D.M., and Wyn-Jones, E., Langmuir, 1993, vol. 9, p. 110.
Gharibi, H., Razavizadeh, B.M., and Rafadi, A.A., Colloids Surf. A, 1998, vol. 136, p. 123.
Chauhan, M.S., Kumar, G., Kumar, A., and Chauhan, S., Colloids Surf., 2000, vol. 166, p. 51.
Ruiz, C.C., J. Colloid Interface Sci., 2000, vol. 221, p. 262.
Lee, D.J. and Huang, W.H., Colloid Polymer Sci., 1996, vol. 274, p. 160.
Mukerjee, P., Adv. Colloid Interface Sci., 1961, vol. 1, p. 241.
Atwood, D. and Florence, A.T., Surfactants Systems: Their Chemistry, Pharmacy, and Biology, New York: Chapman and Hall, 1983.
Bovkun, O.P. and Markina, Z.N., Advances in Colloid Chemistry, Rehbinder, P.A. and Fuks, G.I., Eds., Moscow: Nauka, 1973.
Sugihara, G. and Hisatomi, M., J. Colloid Interface Sci., 1999, vol. 219, p. 31.
Sulthana, S.B., Bhat, S.G.T., and Rakşit, A.K., Colloids Surf. A, 1996, vol. 111, p. 57.
Lumry, R. and Rajender, S., Biopolymers, 1970, vol. 9, p. 1125.
Author information
Authors and Affiliations
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Akbaş, H., Kartal, Ç. Conductometric studies of hexadecyltrimethylammonium bromide in aqueous solutions of ethanol and ethylene glycol. Colloid J 68, 125–130 (2006). https://doi.org/10.1134/S1061933X06020013
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X06020013