Abstract
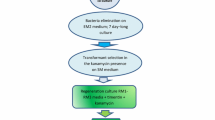
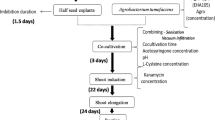
Agrobacterium tumefaciens was used to transform fiber flax with the pBITUBA8 plasmid carrying the mutant α-tubulin gene imparting resistance to dinitroaniline herbicides and the nptII selective marker gene imparting resistance to kanamycin. The transformants were selected in parallel on media containing kanamycin and trifluralin (a dinitroaniline herbicide). The transgenic nature of the resultant regenerants resistant to dinitroaniline herbicides was confirmed by means of Southern blotting and polymerase chain reaction (PCR) analysis using specific probes for the ntpII gene and the gene of α-tubulin.
Similar content being viewed by others
References
Karpets’, I.P., Karpets’, A.I., and Dinnik, O.V., New Method of Fiber Detection in Flax Stems for Selection and Genetic Studies, in Genetika i selektsiya v Ukraini na mezhi tisyacholit’ (Genetics and Selection in Ukraine at the Turn of the Century), Kiiv: Logos, 2001, vol. 3, pp. 68–70.
Molin, W.T. and Khan, R.A., Mitotic Disrupter Herbicide: Recent Advances and Opportunities, Herbicide Activity: Toxicology, Biochemistry and Molecular Biology, Roe, R.M., Ed., Burke: IOS Press, 1997, pp. 143–158.
Vaughn, K.C, Anticytoskeletal Herbicides, Plant Microtubules: Potential for Biotechnology, Nick, P., Ed., New York: Springer, 2000, pp. 193–205.
Vaughn, K.C., The Abnormal Cell Plates Formed after Microtubule Disrupter Herbicide Treatment Are Enriched in Callose, Pestic. Biochem. Physiol., 2006, vol. 84, pp. 63–71.
Yemets, A.I. and Blyum, Ya.B., Plant Resistance to Herbicides with Antimicrotubular Activity: From Natural Mutants to Transgenic Plants, Fiziol. Rastenii, 1999, vol. 46, no. 6, pp. 899–907.
Morejohn, L.C. and Fosket, D.E., The Biochemistry of Compounds with Anti-Microtubule Activity in Plant Cells, Pharm. Ther., 1991, vol. 51, pp. 217–230.
Fosket, D.E. and Morejohn, L.C., Structural and Functional Organization of Tubulin, Ann. Rev. Plant Physiol. Plant. Mol. Biol., 1992, vol. 43, pp. 201–240.
Bajer, A.S. and Molè-Bajer, J., Drugs with Colchicine-Like Effects That Specifically Disassemble Plant but not Animal Microtubules, Ann. N.Y. Acad. Sci., 1986, vol. 466, pp. 767–784.
Blume, Ya.B., Yemets, A.I., Nyporko, A.Yu., and Baird, W.V., Structural Modeling of Plant α-Tubulin Interaction with Dinitroanilines and Phosphoroamidates, Cell Biol. Int., 2003, vol. 27, pp. 171–174.
Anthony, R., Waldin, T., Ray, J., et al., Herbicide Resistance Caused by Spontaneous Mutation of the Cytoskeletal Protein Tubulin, Nature, 1998, vol. 393, pp. 260–263.
Yamamoto, E., Zeng, L., and Baird, W.V., Tubulin Missense Mutations Correlate with Antimicrotubule Drug Resistance in Eleusine indica, Plant Cell, 1998, vol. 10, pp. 297–308.
Radchuk, V.V., Sreenivasulu, N., Blume, Y., and Weschke, W., Distinct Tubulin Genes Are Differentially Expressed during Barley Grain Development, Physiol. Plant., 2007, vol. 131, pp. 571–580.
Toepfer, R., Maas, C., Horicke-Grandpierre, C., et al., Expression Vectors for High-Level Gene Expression in Dicotyledonous and Monocotyledonous Plants, Meth. Enzymol., 1993, vol. 217, pp. 67–78.
Höfgen, R. and Willmitzer, L., Biochemical and Genetic Analysis of Different Patatin Isoforms Expressed in Various Organs of Potato (Solanum tuberosum), Plant Sci., 1990, vol. 66, pp. 221–230.
Hood, E.E., Gelvin, S.B., Melchers, S., and Hoekema, A., New Agrobacterium Helper Plasmids for Gene Transfer to Plants (EHA105), Trans. Res., 1993, vol. 2, pp. 208–218.
Holsters, M., de Waele, D., Depicker, A., et al., Transfection and Transformation of Agrobacterium tumefaciens, Mol. Gen. Genet., 1978, vol. 163, pp. 181–187.
Yemets, A.I., Kundel’chuk, O.P., Smertenko, A.P., et al., Transfer of Amiprophosmethyl-Resistance from a Nicotiana plumbaginifolia Mutant by Somatic Hybridization, Theor. Appl. Genet., 2000, vol. 100, pp. 847–857.
Yemets, A.I., Klimkina, L.A., Tarassenko, L.V., and Blume, Ya.B., Efficient Callus Formation and Plant Regeneration from Dinitroaniline-Resistant and Susceptible Biotypes of Eleusine indica (L.), Plant Cell Rep., 2003, vol. 21, pp. 503–510.
Sambrook, J. and Russell, D.W., Molecular Cloning: A Laboratory Manual, New York: Cold Spring Harbour Lab., 2001.
Baer, O.A., Baer, G.Ya., Yemets, A.I., and Blume, Ya.B., In vitro Culture and Regeneration Ability of Different Flax Varieties of Ukrainian Selections with Different Wind Resistance, Fiziol. Biokhim. Kul’t. Rast., 2004, vol. 36, no. 1, pp. 48–54.
Draper, J., Scott, P., Armitage, F., and Wolden, R., Plant Genetic Transformation and Gene Expression, Oxford: Academic, 1988.
Murashige, T. and Skoog, F., A Revised Medium for Rapid Growth and Bioassay with Tobacco Tissue Cultures, Physiol. Plant., 1962, vol. 15, pp. 473–497.
Radchuk, V.V., Kloke, E., Radchuk, R.I., et al., Production of Transgenic Rapeseed Plants (Brassisa napus L.) by Transformation with Agrobacterium tumefaciens, Russ. J. Genet., 2000, vol. 36, no. 7, pp. 932–941.
Radchuk, V.V., Sreenivasulu, N., Radchuk, R.I., et al., The Methylation Cycle and Its Possible Function in Barley Endosperm Development, Plant. Mol. Biol., 2005, vol. 59, pp. 289–307.
Polyakov, A.V., Chirkizova, O.F., Kalyaeva, M.A., et al., Transformation of Flax Plants, Fiziol. Rastenii, 1998, vol. 45, no. 6, pp. 882–887.
Dong, J.Z. and McHughen, A., Patterns of Transformation Intensity on Flax Hypocotyls Inoculated with Agrobacterium tumefaciens, Plant Cell Rep., 1991, vol. 10, pp. 555–560.
Erdelska, O., Kobeticova, D., and Pretova, A., The in vitro Development of Excised Flax Embryos, Biologia, 1973, vol. 28, pp. 235–239.
Gamborg, O.L. and Shyluk, J.P., Tissue Culture, Protoplasts and Morphogenesis in Flax, Bot. Gaz., 1976, vol. 137, pp. 151–158.
Millam, S., Obert, B., and Pret’ova, A., Plant Cell and Biotechnology Studies in Linum usitatissimum—a Review, Plant Cell Tissue Org. Cult., 2005, vol. 82, pp. 93–103.
Lamblin, F., Aime, A., Hano, C., et al., The Use of the Phosphomannose Isomerase Gene as Alternative Selectable Marker for Agrobacterium-Mediated Transformation of Flax (Linum usitatissimum), Plant Cell Rep., 2007, vol. 26, pp. 765–772.
Jordan, M.C. and McHughen, A., Glyphosate Tolerant Flax Plants from Agrobacterium Mediated Gene Transfer, Plant Cell Rep., 1988, vol. 7, pp. 281–284.
Hano, C., Martin, I., Fliniaux, O., et al., Pinoresinol-Lariciresinol Reductase Gene Expression and Secoisolariciresinol Diglucoside Accumulation in Developing Flax (Linum usitatissimum) Seeds, Planta, 2006, vol. 224, pp. 1291–1301.
McHughen, A., Agrobacterium Mediated Transfer of Chlorsulfuron Resistance to Commercial Flax Cultivars, Plant Cell Rep., 1989, vol. 8, pp. 445–449.
McHughen, A., Jordan, M., and Feist, G., A Preculture Period Prior to Agrobacterium tumefaciens Inoculation Increases Production of Transgenic Plants, J. Plant. Physiol., 1989, vol. 135, pp. 245–248.
Dong, J.Z. and McHughen, A., Transgenic Flax Plants from Agrobacterium tumefaciens Transformation-Incidence of Chimeric Regenerants and Inheritance of Transgenic Plants, Plant Sci., 1993, vol. 91, pp. 139–148.
Ling, H.Q. and Binding, H., Transformation in Protoplast Cultures of Linum usitatissimum and L. suffruticosum Mediated with PEG and with Agrobacterium tumefaciens, J. Plant Physiol., 1997, vol. 151, pp. 479–488.
Dong, J.Z. and McHughen, A., An Improved Procedure for Production of Transgenic Flax Plants Using Agrobacterium tumefaciens, Plant Sci., 1993, vol. 88, pp. 61–71.
Yemets, A.I., Strashnyuk, N.M., and Blume, Ya.B., Plant Mutants and Somatic Hybrids with Resistance to Trifluralin, Cell Biol. Int., 1997, vol. 21, pp. 912–914.
Basiran, N., Armitage, P., Scott, R.J., and Draper, J., Genetic Transformation of Flax (Linum usitatissimum) by Agrobacterium tumefaciens Regeneration of Transformed Shoots via a Callus Phase, Plant Cell Rep., 1987, vol. 6, pp. 396–399.
Yemets, A.I., Kundelchuk, O.P., Smertenko, A.P., et al., Somatic Hybrids of Higher Plants Obtained from Amiprophosmethyl-Resistant Mutant of Nicotiana plumbaginifolia L., Russ. J. Genet., 1996, p. 32, no. 8, pp. 1104–1111.
Yemets, A.I., Blyum, Ya.B., Smertenko, A.P., et al., Obtaining of γ-Hybrids of Higher Plants with Mutant Beta-Tubulin, Dokl. Akad. Nauk, 1997, vol. 353, no. 4, pp. 557–561.
Blume, Ya.B, Kundelchuk, O.P, Solodushko, V.G, et al., Asymmetric Somatic Hybrids of Higher Plants Resistant to Trifluralin, in Proc. Int. Symp. Weed Crop Resist. to Herbicides, De, R., et al., Eds., Cordoba: Univ. Cordoba, 1996, pp. 182–185.
Luduena, R.F., Multiple Forms of Tubulin: Different Gene Products and Covalent Modifications, Inter. Rev. Cyt., 1998, vol. 178, pp. 207–276.
De Buck, S. and Depicker, A., Gene Expression and Level of Expression, Handbook of Plant Biotechnology, Christou, P. and Klee, H., Eds., Chichester: Wiley, 2004, pp. 331–345.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.I. Yemets, O.A. Bayer, V.V. Radchuk, Ya.B. Blume, 2009, published in Genetika, 2009, Vol. 45, No. 10, pp. 1377–1385.
Rights and permissions
About this article
Cite this article
Yemets, A.I., Bayer, O.A., Radchuk, V.V. et al. Agrobacterium-mediated transformation of flax with a mutant tubulin gene responsible for resistance to dinitroaniline herbicides. Russ J Genet 45, 1215–1222 (2009). https://doi.org/10.1134/S1022795409100093
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795409100093