Abstract
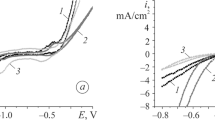
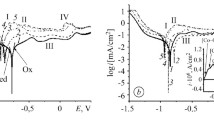
A study is performed of the effect pretreatment with solutions of benzoic acid in an organic solvent has on the result from the electrochemical oxidation of amorphous alloy Fe70Cr15B15 in an ionic liquid 1‑butyl-3-methylimidazolium tetrafluoroborate. It is established that the nature and properties of surface oxide films affect the result from the electrochemical oxidation of the alloy in an ionic liquid. Partial removal of oxide films by a solution of benzoic acid and subsequent electrooxidation in an ionic liquid reveals a nano-cellular structure on the electrode’s surface. It is shown that preliminarily treating the alloy with a saturated solution of benzoic acid in acetone results in a considerable anodic shift in the corrosion potential and the formation of a more effective protective coating.



Similar content being viewed by others
REFERENCES
W. B. Nowak and B. A. Okorie, Corrosion 38, 314 (1982). https://doi.org/10.5006/1.3621691
V. Paredes, E. Salvagni, E. Rodriguez, et al., J. Mater. Sci.: Mater. Med. 25, 311 (2014). https://doi.org/10.1007/s10856-013-5083-2
C. Wang, H. Zheng, H. Ding, et al., J. Alloys Compd. 766, 649 (2018). https://doi.org/10.1016/j.jallcom.2018.06.368
M. Huber, G. Reinisch, G. Trettenhahn, et al., Acta Biomater. 5, 172 (2009). https://doi.org/10.1016/j.actbio.2008.07.032
Stainless Steels for Medical and Surgical Applications, Proceedings of the Symposium, Ed. by G. L. Winters and M. J. Nutt (ASTM Int., 2003).
S. Sowmiah, V. Srinivasadesikan, M. C. Tseng, et al., Molecules 14, 3780 (2009). https://doi.org/10.3390/molecules14093780
Y. Zhijie, L. Jinfu, H. Shunrong, et al., Mater. Trans. 44, 709 (2003). https://doi.org/10.2320/matertrans.44.709
S. B. Gabriel, J. V. P. Panaino, I. D. Santos, et al., J. Alloys Compd. 536S, S208 (2012). https://doi.org/10.1016/j.jallcom.2011.11.035
O. Lebedeva, I. Kudryavtsev, D. Kultin, et al., J. Phys. Chem. C 118, 21293 (2014). https://doi.org/10.1021/jp507319r
K. B. Kalmykov, N. E. Dmitrieva, O. K. Lebedeva, N. V. Root, D. Yu. Kultin, and L. M. Kustov, Russ. Chem. Bull. 65, 2801 (2016). https://doi.org/10.1007/s11172-016-1659-6
H. M. El-Kashlan, Asian J. Chem. 23, 5235 (2011).
ACKNOWLEDGMENTS
The authors thank V.S. Snytko, I.I. Kuznetsova, and I.V. Balykova for their help in preparing this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lebedeva, O.K., Kultin, D.Y., Zakharov, A.N. et al. Electrochemical Behavior of an Amorphous Alloy in an Ionic Liquid and in Aqueous Media. Russ. J. Phys. Chem. 94, 2379–2381 (2020). https://doi.org/10.1134/S0036024420110205
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420110205