Abstract
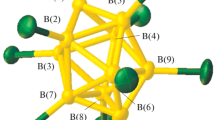
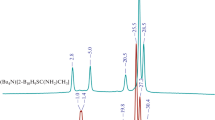
The structures of cesium and tetraphenylphosphonium polyborates obtained in the course of mild oxidation of the closo-decaborate anion [B10H10]2– in water with a cerium(IV) salt to form Cs2[trans-B20H18] as the main product and in the course of long-term isomerization of the [trans-B20H18]2– anion under the action of UV irradiation to form (Ph4P)2[iso-B20H18] as the main product have been studied. The compounds have been isolated as solvates Cs[B5O6(OH)4]·2H2O and PPh4[B5O6(OH)4]·2DMF·H2O from the filtrates obtained after removing the target compounds from the reaction solutions. The formation of polyborates is explained by the processes of degradation of boron cluster anions in reaction solutions over time.





Similar content being viewed by others
REFERENCES
E. L. Muetterties and W. H. Knoth, Polyhedral Boranes (Marcel Dekker, New York, 1968).
I. B. Sivaev, V. I. Bregadze, S. Sjöberg, et al., Collect. Czech. Chem. Commun. 87, 679 (2002).
I. B. Sivaev, A. V. Prikaznov, and D. Naoufal, Collect. Czech. Chem. Commun. 75, 1149 (2010).
K. Yu. Zhizhin, A. P. Zhdanov, N. T. Kuznetsov, et al., Russ. J. Inorg. Chem. 55, 2089 (2010).
S. E. Korolenko, A. S. Kubasov, L. V. Goeva, et al., Inorg. Chim. Acta 527, 120587 (2021). https://doi.org/10.1016/j.ica.2021.120587
Y. Zhang, L. Yang, and L. Wang, Chem. Int. Ed. 58, 8145 (2019).
S. E. Korolenko, K. P. Zhuravlev, V. I. Tsaryuk, et al., J. Lumin. 237, 118156 (2021). https://doi.org/10.1016/j.jlumin.2021.118156
V. V. Avdeeva, E. A. Malinina, A. V. Churakov, et al., Polyhedron 169, 144 (2019). https://doi.org/10.1016/j.poly.2019.01.051
V. V. Avdeeva, I. N. Polyakova, L. V. Goeva, et al., Russ. J. Inorg. Chem. 61, 302 (2016).
E. A. Malinina, S. E. Korolenko, A. P. Zhdanov, et al., J. Clust. Chem. 32, 755 (2021). https://doi.org/10.1007/s10876-020-01840-5
S. E. Korolenko, V. V. Avdeeva, E. A. Malinina, et al., Russ. J. Inorg. Chem. 66, 1350 (2021). https://doi.org/10.1134/S0036023621090047
Y. Sadikin, E. Didelot, Z. Łodziana, and R. Černý, Dalton Trans. 47, 5843 (2018).
Z. Zhang, Y. Zhang, Z. Li, et al., Eur. J. Inorg. Chem. 981 (2018).
E. A. Malinina, I. K. Kochneva, V. V. Avdeeva, et al., Russ. J. Inorg. Chem. 64, 1210 (2019).
A. H. Norman and A. Kaczmarczyk, Inorg. Chem. 13, 2316 (1974).
A. E. Dziova, V. V. Avdeeva, I. N. Polyakova, et al., Dokl. Chem. 440, 253 (2011).
V. V. Avdeeva, A. E. Dziova, I. N. Polyakova, et al., Russ. J. Inorg. Chem. 58, 657 (2013).
E. F. Safronova, V. V. Avdeeva, I. N. Polyakova, et al., Dokl. Chem. 452, 240 (2013).
V. V. Avdeeva, A. V. Vologzhanina, L. V. Goeva, et al., Inorg. Chim. Acta 428, 154 (2015).
V. V. Avdeeva, I. N. Polyakova, A. V. Churakov, et al., Polyhedron 162, 65 (2019).
V. V. Avdeeva, E. A. Malinina, L. V. Goeva, et al., Dokl. Chem. 474, 141 (2017).
J. Miao, Y. Nie, H. Chen, et al., Z. Naturforsch. 66B, 387 (2011).
Y. Nie, J.-L. Miao, C.-H. Hu, et al., Polyhedron 31, 607 (2012).
G. A. Abakumov, A. V. Piskunov, V. K. Cherkasov, et al., Russ. Chem. Rev. 87, 393 (2018). https://doi.org/10.1070/RCR4795
S. I. Pechenyuk, D. P. Domonov, and A. N. Gosteva, Russ. J. Gen. Chem. 91, 1834 (2021). https://doi.org/10.1134/S1070363221090310
V. V. Bardin, S. A. Prikhod’ko, M. M. Shmakov, et al., Russ. J. Gen. Chem. 90, 50 (2020). https://doi.org/10.1134/S1070363220010089
A. P. Topnikova and E. L. Belokoneva, Russ. Chem. Rev. 88, 204 (2019). https://doi.org/10.1070/RCR4835
V. V. Avdeeva, E. A. Malinina, A. V. Vologzhanina, et al., Inorg. Chim. Acta 509, 119693 (2020). https://doi.org/10.1016/j.ica.2020.119693
H. C. Miller, N. E. Miller, E. L. Muetterties, et al., J. Am. Chem. Soc. 85, 3885 (1963). https://doi.org/10.1021/ja00906a033
B. L. Chamberland and E. L. Muetterties, Inorg. Chem. 3, 1450 (1964). https://doi.org/10.1021/ic50020a025
G. M. Sheldrick, Acta Crystallogr., Sect. C 71, 3 (2015).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009).
A. Kaczmarczyk, R. D. Dobrott, and W. N. Lipscomb, Proc. Nat. Acad. Sci. U.S.A. 48, 729 (1962).
M. F. Hawthorne, R. L. Pilling, P. F. Stokely, and P. M. Garrett, J. Am. Chem. Soc. 85, 3704 (1963).
Z. B. Curtis, C. Young, R. Dickerson, and A. Kaczmarczyk, Inorg. Chem. 13, 1760 (1974).
F. Li, K. Shelly, C. B. Knobler, and M. F. Hawthorne, Angew. Chem., Int. Ed. 37, 1865 (1998).
V. V. Avdeeva, M. I. Buzin, E. A. Malinina, et al., CrystEngComm 17, 8870 (2015).
V. V. Avdeeva, E. A. Malinina, K. Yu. Zhizhin, et al., J. Struct. Chem. 60, 692 (2019). https://doi.org/10.1134/S0022476619050020
V. V. Avdeeva, A. S. Kubasov, S. E. Korolenko, et al., Russ. J. Inorg. Chem. 67, 8 (2022). https://doi.org/10.31857/S0044457X22050026
Xuetao Xu, Kanyi Liang, and Yirou Lin, Z. Anorg. Allg. Chem. 640, 110 (2014).
M. A. Beckett, S. J. Coles, and P. N. Horton, J. Cluster Sci. 28, 2087 (2017).
Sa-Ying Li and Zhi-Hong Liu, J. Therm. Anal. Calorim. 126, 913 (2016).
Yang Yang, DongSheng Fu, GuoFa Li, and Yun Zhang, Z. Anorg. Allg. Chem. 639 (2013).
G. A. Abakumov, A. V. Piskunov, V. K. Cherkasov, et al., Russ. Chem. Rev. 87, 393 (2018). https://doi.org/10.1070/RCR4795
S. E. Korolenko, L. V. Goeva, A. S. Kubasov, et al., Russ. J. Inorg. Chem. 65, 846 (2020). https://doi.org/10.1134/S0036023620060091
I. B. Sivaev, Russ. J. Inorg. Chem. 65, 1854 (2020). https://doi.org/10.1134/S0036023620120165
E. Yu. Matveev, I. V. Novikov, A. S. Kubasov, et al., Russ. J. Inorg. Chem. 66, 187 (2021). https://doi.org/10.1134/S0036023621020121
I. N. Klyukin, A. V. Kolbunova, N. A. Selivanov, et al., Russ. J. Inorg. Chem. 66, 1798 (2021). https://doi.org/10.1134/S003602362112007X
A. V. Burdenkova, A. P. Zhdanov, I. N. Klyukin, et al., Russ. J. Inorg. Chem. 66, 1616 (2021). https://doi.org/10.1134/S0036023621110036
ACKNOWLEDGMENTS
Analytical studies were carried out at the Center for Collective Use of the Physical Methods of Investigation of the Kurnakov Institute RAS within the framework of the State Assignment of the Kurnakov Institute RAS.
Funding
The work was carried out within the framework of the State Assignment of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences in the field of fundamental scientific research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Kubasov, A.S., Novikov, I.V., Starodubets, P.A. et al. Formation of Polyborates during Dimerization of the closo-Decaborate Anion and Isomerization of the Octadecahydroeicosaborate Anion. Russ. J. Inorg. Chem. 67, 984–991 (2022). https://doi.org/10.1134/S0036023622070130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622070130