Abstract
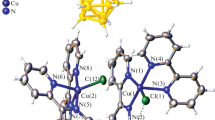
Copper(II) complexation of the [B10H10]2– anion has been studied in the presence of 2,2′-bipyridyl (bipy) in organic solvents. The reaction between CuCl, bipy, and (Et3NH)[Ag[B10H10]] in DMF leads to copper(II) complex [Cu2(bipy)4(µ-CO3)][B10H10]·2DMF·H2O. The copper complexation reaction has been performed under the redox conditions Cu(I) → Cu(II) in the presence of silver(I) compounds. When [Cu2(bipy)4(µ-CO3)][B10H10]·2DMF·H2O has been heated in DMSO, the monosubstituted derivative [2-B10H9OH]2– has been isolated as complex [Cu2(bipy)4(µ-CO3)][2-B10H10O0.17]·2DMSO·H2O consisting of the [B10H10]2– anion and its monosubstituted derivative [2-B10H9OH]2– cocrystallized in the 0.83:0.17 ratio. The metal-promoted process of exopolyhedral substitution of terminal hydrogen atoms in the [B10H10]2¬ anion in the presence of Cu(II) compounds has been discussed. Complexes synthesized have been studied by elemental analysis, IR, 1H and 11B NMR spectroscopy; the X-ray diffraction studies were performed for [Cu2(bipy)4(µ-CO3)][B10H10]·2DMF·H2O and [Cu2(bipy)4(µ-CO3)][2-B10H10O0.17]·2DMSO·H2O.




Similar content being viewed by others
References
K. M. Krishnan Fundamentals and Applications of Magnetic Materials (Oxford University Press, Oxford, 2016).
E. L. Muetterties and W. H. Knoth Polyhedral Boranes (Dekker, New York, 1968).
N. N. Greenwood and A. Earnshaw Chemistry of the Elements, 2nd ed (Butterworth-Heinemann, Oxford, 1997).
Boron Science: New Technologies and Applications, ed. by N. S. Hosmane (CRC Press, Boca Raton, 2012).
R. N. Grimes Carboranes, 3rd ed (Academic Press, US, 2016).
M. Scholz and E. Hey-Hawkins (2011). Chem. Rev. 111, 7035.
N. S. Hosmane and R. Eagling Handbook of Boron Science with Applications in Organometallics, Catalysis, Materials and Medicine (World Scientific, Singapore, 2019).
C. Viñas and F. Teixidor (2013). Future. Med. Chem. 5, 617.
R. Núñez, I. Romero, F. Teixidor, and C. Viñas (2016). Chem. Soc. Rev. 45, 5147.
J. Poater, M. Solà, C. Viñas, and F. Teixidor (2014). Angew. Chem. Int. Ed. 53, 12191.
R. B. King (2001). Chem. Rev. 101, 1119.
Z. Chen and R. B. King (2005). Chem. Rev. 105, 3613.
K Yu Zhizhin, A. P. Zhdanov, and N. T. Kuznetsov (2010). Russ. J. Inorg. Chem. 55, 2089.
I. B. Sivaev, A. V. Prikaznov, and D. Naoufal (2010). Collect. Czech. Chem. Commun. 75, 1149.
I. B. Sivaev, V. I. Bregadze, and S. Sjöberg (2002). Collect. Czech. Chem. Commun. 67, 679.
V. V. Avdeeva, E. A. Malinina, I. B. Sivaev, V. I. Bregadze, and N. T. Kuznetsov (2016). Crystals 6, 60.
E. A. Malinina, V. V. Avdeeva, L. V. Goeva, and N. T. Kuznetsov (2010). Russ. J. Inorg. Chem. 55, 2148.
V. V. Avdeeva, E. A. Malinina, and N. T. Kuznetsov (2017). Russ. J. Inorg. Chem. 62, 1673.
L. N. Goswami, L. Ma, Sh Chakravarty, Q. Cai, S. S. Jalisatgi, and M. F. Hawthorne (2013). Inorg. Chem. 52, 1694.
J. Plesek (1992). Chem. Rev. 92, 269.
I. B. Sivaev, V. I. Bregadze, and N. T. Kuznetsov (2002). Russ. Chem. Bull. 51, 1362.
I. B. Sivaev and V. I. Bregadze (2009). Eur. J. Inorg. Chem. 11, 1433.
F. Teixidor, C. Vinas, and A. Demonceau (2003). Pure Appl. Chem. 75, 1305.
I. N. Klyukin, A. P. Zhdanov, A. Y. Bykov, V. M. Retivov, K Yu Zhizhin, and N. T. Kuznetsov (2018). Russ. J. Inorg. Chem. 63, 213.
A. V. Prikaznov, V. I. Bragin, M. N. Davydova, I. B. Sivaev, and V. I. Bregadze (2007). Collect. Czech. Chem. Commun. 72, 1689.
I. N. Klyukin, A. S. Novikov, A. P. Zhdanov, K Yu Zhizhin, and N. T. Kuznetsov (2019). Russ. J. Inorg. Chem. 64, 1825.
K Yu Zhizhin, O. O. Vovk, E. A. Malinina, V. N. Mustyatsa, K Yu Zhizhin, and N. T. Kuznetsov (2005). Russ. J. Inorg. Chem. 50, 243.
I. N. Klyukin, V. V. Voinova, N. A. Selivanov, A. P. Zhdanov, K Yu Zhizhin, and N. T. Kuznetsov (2018). Russ. J. Inorg. Chem. 63, 1546.
E. F. Safronova, V. V. Avdeeva, I. N. Polyakova, A. V. Vologzhanina, L. V. Goeva, E. A. Malinina, and N. T. Kuznetsov (2013). Doklady Chem. 452, 240.
V. V. Avdeeva, A. V. Vologzhanina, L. V. Goeva, E. A. Malinina, and N. T. Kuznetsov (2015). Inorg. Chim. Acta 428, 154.
V. V. Avdeeva, I. N. Polyakova, A. V. Churakov, A. V. Vologzhanina, E. A. Malinina, K Yu Zhizhin, and N. T. Kuznetsov (2019). Polyhedron 162, 65.
V. V. Avdeeva, E. A. Malinina, A. V. Vologzhanina, I. B. Sivaev, and N. T. Kuznetsov (2020). Inorg. Chim. Acta 509, 119693.
H. Abbas, C. Streb, A. L. Pickering, A. R. Neil, D.-L. Long, and L. Cronin (2008). Cryst. Growth Des. 8, 635.
V. V. Avdeeva, E. A. Malinina, A. V. Churakov, I. N. Polyakova, and N. T. Kuznetsov (2019). Polyhedron 169, 144.
I. K. Kochneva, V. V. Avdeeva, L. V. Goeva, E. A. Malinina, and N. T. Kuznetsov (2018). Russ. J. Inorg. Chem. 63, 591.
E. A. Malinina, I. K. Kochneva, I. N. Polyakova, V. V. Avdeeva, G. A. Buzanov, N. N. Efimov, E. A. Ugolkova, V. V. Minin, and N. T. Kuznetsov (2018). Inorg. Chim. Acta. 479, 249.
E. A. Malinina, I. K. Kochneva, I. N. Polyakova, V. V. Avdeeva, L. V. Goeva, V. V. Minin, E. A. Ugolkova, and N. T. Kuznetsov (2018). Inorg. Chim. Acta. 477, 284.
E. A. Malinina, I. K. Kochneva, V. V. Avdeeva, L. V. Goeva, A. S. Kubasov, and N. T. Kuznetsov (2019). Russ. J. Inorg. Chem. 64, 1210.
A. E. Dziova, V. V. Avdeeva, I. N. Polyakova, O. N. Belousova, E. A. Malinina, and N. T. Kuznetsov (2011). Doklady Chem. 440, 253.
V. V. Avdeeva, A. E. Dziova, I. N. Polyakova, L. V. Goeva, E. A. Malinina, and N. T. Kuznetsov (2013). Russ. J. Inorg. Chem. 58, 657.
V. V. Drozdova, E. A. Malinina, O. N. Belousova, I. N. Polyakova, and N. T. Kuznetsov (2008). Russ. J. Inorg. Chem. 53, 1024.
APEX2, Version 2014.11-0, Bruker AXS Inc. (Madison, Wisconsin, USA).
SADABS, Version 2014/5, Bruker AXS Inc. (Madison, Wisconsin, USA).
SAINT, Version V8.34A, Bruker AXS Inc. (Madison, Wisconsin, USA).
G. M. Sheldrick (1990). Acta Cryst. A46, 467.
SHELXTL, Version 6.14, Bruker AXS Inc. (Madison, Wisconsin, USA).
A. E. Dziova, V. V. Avdeeva, I. N. Polyakova, E. A. Malinina, A. V. Rotov, N. N. Efimov, E. A. Ugolkova, V. V. Minin, and N. T. Kuznetsov (2013). Russ. J. Inorg. Chem. 58, 1527.
V. V. Avdeeva, A. E. Dziova, I. N. Polyakova, E. A. Malinina, L. V. Goeva, and N. T. Kuznetsov (2015). Inorg. Chim. Acta 430, 74.
A. V. Vologzhanina, A. A. Korlyukov, V. V. Avdeeva, I. N. Polyakova, E. A. Malinina, and N. T. Kuznetsov (2013). J. Phys. Chem. A 117, 13138.
V. V. Avdeeva, A. E. Dziova, I. N. Polyakova, L. V. Goeva, E. A. Malinina, and N. T. Kuznetsov (2011). Dokl Chem. 437, 79.
A. E. Dziova, V. V. Avdeeva, I. N. Polyakova, E. A. Malinina, A. V. Rotov, N. N. Efimov, V. V. Minin, and N. T. Kuznetsov (2012). Doklady Chem. 442, 1.
I. K. Kochneva, I. N. Polyakova, V. V. Avdeeva, N. N. Efimov, E. A. Ugolkova, V. V. Minin, E. A. Malinina, and N. T. Kuznetsov (2017). Doklady Chem. 474, 137.
V. V. Avdeeva, E. A. Malinina, K Yu Zhizhin, and N. T. Kuznetsov (2020). Russ. J. Inorg. Chem. 65, 335.
Acknowledgments
The X-ray diffraction studies were performed by Irina Polyakova (deceased) at the Kurnakov Institute and were described by Dr. Sergey Nefedov (the Kurnakov Institute). The IR spectra of the compounds were measured by Ph. D. Lyudmila V. Goeva (the Kurnakov Institute).
Funding
The studies were performed within the framework of the State Assignment of the Kurnakov Institute (IGIC RAS) in the field of fundamental scientific research and was supported in part by the Russian Foundation for Basic Research (Russia), grant no. 20-03-00763. Synthesis of compounds was supported by the Council for Grants of the President of RF for State Support of scientific research performed by Young Russian Scientists (Grant MD-265.2019.3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malinina, E.A., Korolenko, S.E., Zhdanov, A.P. et al. Metal-Promoted Exopolyhedral Substitution of Terminal Hydrogen Atoms in the Closo-Decaborate Anion [B10H10]2– in the Presence of Copper(II): Formation of the Substituted Derivative [2-B10H9OH]2–. J Clust Sci 32, 755–763 (2021). https://doi.org/10.1007/s10876-020-01840-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01840-5