Abstract
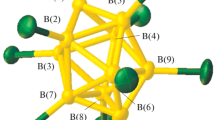
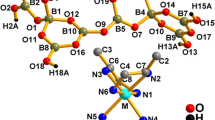
The reactivity of the decahydro-closo-decaborate anion in the complexation of metals being among intermediates acids in the concept of the Pearson’s theory of hard and soft acids and bases is studied systematically in the presence of competitive ligands L. In continuing these studies, we have synthesized Zn(II) and Cd(II) complexes of the composition [М(DMF)6][B10H10] (M = Zn(II), Cd(II)) and studied them by physicochemical analyses (IR and UV spectroscopy, X-ray powder diffraction). The possibility of their use in solid-phase synthesis of the corresponding trischelate complexes [M(Bipy)3][B10H10] has been found. Complexes [М(DMF)6][B10H10] are formed by the reaction between triethylammonium closo-decaborate with metal nitrates in DMF. The compounds are isostructural with the corresponding Co(II) and Ni(II) complexes [М(DMF)6][B10H10]. Specific interactions have been found between the BH groups of the boron clusters and CH groups of the DMF molecules. According to the data of IR and UV spectroscopy, the complexes contain specific B–H···C–H interactions. The corresponding tris-chelate complexes of the metals [M(Bipy)3][B10H10] are also isostructural.
Similar content being viewed by others
References
E. A. Malinina, V. V. Avdeeva, L. V. Goeva, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 55, 2148 (2010). doi 10.1134/S0036023610140032
Nguyen Duc Van, Thesis (Inst. fur anorganische Chemie der Universitat, Stuttgart, 2009).
V. V. Avdeeva, E. A. Malinina, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 62, 1673 (2017). doi 10.1134/S0036023617130022
V. V. Avdeeva, A. V. Vologzhanina, L. V. Goeva, et al., Anorg. Allg. Chem. 640, 2149 (2014). doi 10.1002/zaac.201400137
V. V. Avdeeva, A. V. Vologzhanina, L. V. Goeva, et al., Dokl. Akad. Nauk 461, 664 (2015). doi 10.7868/S0869565215120129
V. V. Avdeeva, A. V. Vologzhanina, E. A. Malinina, et al., Inorg. Chim. Acta 428, 154 (2015). doi 10.1016/j.ica.2014.12.029
V. V. Avdeeva, I. N. Polyakova, L. V. Goeva, et al., Russ. J. Inorg. Chem. 60, 817 (2015). doi 10.1134/S0036023615070037
V. V. Avdeeva, I. N. Polyakova, L. V. Goeva, et al., Russ. J. Inorg. Chem. 61, 302 (2016). doi 10.1134/S0036023616030037
E. F. Safronova, V. V. Avdeeva, Polyakova I.N., et al., Dokl. Chem. 452, 240 (2013). doi 10.1134/S0012500813110013
A. E. Dziova, V. V. Avdeeva, I. N. Polyakova, et al., Dokl. Chem. 440, 253 (2011). doi 10.1134/S0012500811090035
A. D. Kayumov, K. A. Solntsev, N. T. Kuznetsov, et al., Zh. Neorg. Khim. 33, 1771 (1988).
Yu. L. Gaft, N. T. Kuznetsov, and L. M. Sukova, Zh. Neorg. Khim. 28, 162 (1983).
A. D. Kayumov, L. V. Goeva, N. T. Kuznetsov, et al., Zh. Neorg. Khim. 33, 1936 (1988).
A. D. Kayumov, K. A. Solntsev, L. V. Goeva, et al., Zh. Neorg. Khim. 35, 1729 (1990).
I. Tiritiris and Th. Schleid, Z. Anorg. Allg. Chem. 631, 1593 (2005). doi 10.1002/zaac.200500093
I. Tiritiris and Th. Schleid, Z. Anorg. Allg. Chem. 634, 317 (2008). doi 10.1002/zaac.200700399
E. A. Malinina, V. V. Drozdova, L. V. Goeva, et al., Russ. J. Inorg. Chem. 52, 854 (2007). doi 10.1134/S003602360706006X
H. C. Miller, N. E. Miller, and E. L. Muetterties, J. Am. Chem. Soc. 85, 3885 (1963). doi 10.1021/ja00906a033
V. V. Avdeeva, I. N. Polyakova, L. V. Goeva, et al., Inorg. Chim. Acta 451, 129 (2016). doi 10.1016/j.ica.2016.07.016
V. V. Avdeeva, I. N. Polyakova, A. V. Vologzhanina, et al., Russ. J. Inorg. Chem. 61, 1125 (2016). doi 10.1134/S0036023616090023
L. V. Goeva, V. V. Avdeeva, E. A. Malinina, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 63, 1050 (2018). doi 10.1134/S0036023618120148
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.A. Malinina, S.E. Korolenko, L.V. Goeva, G.A. Buzanov, V.V. Avdeeva, N.T. Kuznetsov, 2018, published in Zhurnal Neorganicheskoi Khimii, 2018, Vol. 63, No. 12, pp. 1543–1548.
Rights and permissions
About this article
Cite this article
Malinina, E.A., Korolenko, S.E., Goeva, L.V. et al. Synthesis and Structure of [М(DMF)6][B10H10] (M = Zn(II), Cd(II)) as Precursors for Solid-Phase Synthesis of Trischelate Complexes [М(L)3][B10H10]. Russ. J. Inorg. Chem. 63, 1552–1557 (2018). https://doi.org/10.1134/S0036023618120148
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023618120148