Abstract
Dysregulated proinflammatory cytokine expression may result in the development of severe pathologies, such as rheumatoid arthritis, psoriasis, and neurodegenerative diseases. Transgenic mice and, in particular, those with controllable systemic overexpression of proinflammatory cytokines have recently become an essential instrument to study the molecular mechanisms underlying disease development. Importantly, many of the models are humanized by introducing a human cytokine gene, while leaving or removing the respective endogenous mouse gene. Humanized mice are especially valuable for biomedical research as they provide a relevant model to develop therapies based on blocking the pathogenic activity of a cytokine or to establish the functional significance of genome polymorphisms. The review discusses the available humanized mouse models with overexpression of key proinflammatory cytokines (TNF, IL-1β, and IL-6) and inflammatory cytokines with more specific functions (IL-8, IL-17, and IL-32) and their significance for basic and clinical research.


Similar content being viewed by others
Notes
When describing mouse models with transgenic overexpression of human cytokines, we hereafter use the additional letter h to denote the respective human protein (e.g., hTNF), while the protein name without the additional h refers to the mouse protein.
REFERENCES
Gama Sosa M.A., De Gasperi R., Elder G.A. 2010. Animal transgenesis: An overview. Brain Struct. Funct. 214, 91–109.
Keffer J., Probert L., Cazlaris H., Georgopoulos S., Kaslaris E., Kioussis D., Kollias G. 1991. Transgenic mice expressing human tumour necrosis factor: A predictive genetic model of arthritis. EMBO J. 10, 4025–4031.
Akassoglou K., Probert L., Kontogeorgos G., Kollias G. 1997. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J. Immunol. 158, 438–445.
Isoda K., Kamezawa Y., Tada N., Sato M., Ohsuzu F. 2001. Myocardial hypertrophy in transgenic mice overexpressing human interleukin 1alpha. J. Card. Fail. 7, 355–364.
Kitamura T., Koshino Y., Shibata F., Oki T., Nakajima H., Nosaka T., Kumagai H. 2003. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp. Hematol. 31, 1007–1014.
Yan B.W., Zhao Y.F., Cao W.G., Li N., Gou K.M. 2013. Mechanism of random integration of foreign DNA in transgenic mice. Transgenic Res. 22, 983–992.
Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71.
Quadros R.M., Miura H., Harms D.W., Akatsuka H., Sato T., Aida T., Redder R., Richardson G.P., Inagaki Y., Sakai D., Buckley S.M., Seshacharyulu P., Batra S.K., Behlke M.A., Zeiner S.A., et al. 2017. Easi-CRISPR: A robust method for one-step generation of mice carrying conditional and insertion alleles using long ssDNA donors and CRISPR ribonucleoproteins. Genome Biol. 18, 92.
Scott G.J., Gruzdev A. 2019. Genome editing in mouse embryos with CRISPR/Cas9. Methods Mol. Biol. 1960, 23–40.
Li G., Wu Y., Jia H., Tang L., Huang R., Peng Y., Zhang Y. 2016. Establishment and evaluation of a transgenic mouse model of arthritis induced by overexpressing human tumor necrosis factor alpha. Biol. Open. 5, 418–423.
Carswell E.A., Old L.J., Kassel R.L., Green S., Fiore N., Williamson B. 1975. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. U. S. A. 72, 3666–3670.
Grivennikov S.I., Kuprash D.V., Liu Z.G., Nedospasov S.A. 2006. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: From simple paradigms to complex mechanisms. Int. Rev. Cytol. 252, 129–161.
Kruglov A.A., Kuchmiy A., Grivennikov S.I., Tumanov A.V., Kuprash D.V., Nedospasov S.A. 2008. Physiological functions of tumor necrosis factor and the consequences of its pathologic overexpression or blockade: mouse models. Cytokine Growth Factor Rev. 19, 231–244.
Old L.J. 1988. Tumor necrosis factor. Sci. Am. 258, 59–60, 69–75.
Carballo E., Lai W.S., Blackshear P.J. 1998. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 281, 1001–1005.
Patel H.J., Patel B.M. 2017. TNF-alpha and cancer cachexia: Molecular insights and clinical implications. Life Sci. 170, 56–63.
Zhao Y., Wang Y., Zhu M.S., Han W.M., Li Z., Hong S.F., Yin P., Zhuang G.H., Qi Z.Q. 2018. Expression pattern of tumor necrosis factor-alpha-induced protein 8-like 2 in acute rejection of cardiac allograft. Transplant. Proc. 50, 293–298.
Beutler B.A. 1989. Orchestration of septic shock by cytokines: The role of cachectin (tumor necrosis factor). Prog. Clin. Biol. Res. 286, 219–235.
Sfikakis P.P., Kollias G. 2003. Tumor necrosis factor biology in experimental and clinical arthritis. Curr. Opin. Rheumatol. 15, 380–386.
Yamauchi P.S., Bissonnette R., Teixeira H.D., Valdecantos W.C. 2016. Systematic review of efficacy of anti-tumor necrosis factor (TNF) therapy in patients with psoriasis previously treated with a different anti-TNF agent. J. Am. Acad. Dermatol. 75, 612–618. e6.
Gorth D.J., Shapiro I.M., Risbud M.V. 2018. Transgenic mice overexpressing human TNF-alpha experience early onset spontaneous intervertebral disc herniation in the absence of overt degeneration. Cell Death Dis. 10, 7.
Retser E., Schied T., Skryabin B.V., Vogl T., Kanczler J.M., Hamann N., Niehoff A., Hermann S., Eisenblatter M., Wachsmuth L., Pap T., van Lent P.L., Loser K., Roth J., Zaucke F., et al. 2013. Doxycycline-induced expression of transgenic human tumor necrosis factor alpha in adult mice results in psoriasis-like arthritis. Arthritis Rheum. 65, 2290–2300.
Probert L., Keffer J., Corbella P., Cazlaris H., Patsavoudi E., Stephens S., Kaslaris E., Kioussis D., Kollias G. 1993. Wasting, ischemia, and lymphoid abnormalities in mice expressing T cell-targeted human tumor necrosis factor transgenes. J. Immunol. 151, 1894–1906.
Drutskaya M.S., Efimov G.A., Zvartsev R.V., Chashchina A.A., Chudakov D.M., Tillib S.V., Kruglov A.A., Nedospasov S.A. 2014. Experimental models of arthritis in which pathogenesis is dependent on TNF expression. Biochemistry (Moscow). 79 (12), 1349–1357.
Franciotta D.M., Grimaldi L.M., Martino G.V., Piccolo G., Bergamaschi R., Citterio A., Melzi d’Eril G.V. 1989. Tumor necrosis factor in serum and cerebrospinal fluid of patients with multiple sclerosis. Ann. Neurol. 26, 787–789.
Kim Y.S., Lee K.J., Kim H. 2017. Serum tumour necrosis factor-alpha and interleukin-6 levels in Alzheimer’s disease and mild cognitive impairment. Psychogeriatrics. 17, 224–230.
Zimmerman A.W., Jyonouchi H., Comi A.M., Connors S.L., Milstien S., Varsou A., Heyes M.P. 2005. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr. Neurol. 33, 195–201.
Dinarello C.A. 2018. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281, 8–27.
Nishikawa K., Yoshida M., Kusuhara M., Ishigami N., Isoda K., Miyazaki K., Ohsuzu F. 2006. Left ventricular hypertrophy in mice with a cardiac-specific overexpression of interleukin-1. Am. J. Physiol. Heart Circ. Physiol. 291, H176–H183.
Niki Y., Yamada H., Seki S., Kikuchi T., Takaishi H., Toyama Y., Fujikawa K., Tada N. 2001. Macrophage- and neutrophil-dominant arthritis in human IL-1 alpha transgenic mice. J. Clin. Invest. 107, 1127–1135.
Shaftel S.S., Carlson T.J., Olschowka J.A., Kyrkanides S., Matousek S.B., O’Banion M.K. 2007. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J. Neurosci. 27, 9301–9309.
Hein A.M., Stasko M.R., Matousek S.B., Scott-McKean J.J., Maier S.F., Olschowka J.A., Costa A.C., O’Banion M.K. 2010. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav. Immun. 24, 243–253.
Tu S., Bhagat G., Cui G., Takaishi S., Kurt-Jones E.A., Rickman B., Betz K.S., Penz-Oesterreicher M., Bjorkdahl O., Fox J.G., Wang T.C. 2008. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 14, 408–419.
Marrache F., Tu S.P., Bhagat G., Pendyala S., Osterreicher C.H., Gordon S., Ramanathan V., Penz-Osterreicher M., Betz K.S., Song Z., Wang T.C. 2008. Overexpression of interleukin-1beta in the murine pancreas results in chronic pancreatitis. Gastroenterology. 135, 1277–1287.
Lappalainen U., Whitsett J.A., Wert S.E., Tichelaar J.W., Bry K. 2005. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am. J. Respir. Cell Mol. Biol. 32, 311–318.
Bjorkdahl O., Akerblad P., Gjorloff-Wingren A., Leanderson T., Dohlsten M. 1999. Lymphoid hyperplasia in transgenic mice over-expressing a secreted form of the human interleukin-1beta gene product. Immunology. 96, 128–137.
Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. 1987. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc. Natl. Acad. Sci. U. S. A. 84, 7251–7255.
Crotty S. 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity. 41, 529–542.
Nemeth E., Rivera S., Gabayan V., Keller C., Taudorf S., Pedersen B.K., Ganz T. 2004. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 113, 1271–1276.
Irwin M.R. 2011. Inflammation at the intersection of behavior and somatic symptoms. Psychiatr. Clin. North Am. 34, 605–620.
Behm B., Babilas P., Landthaler M., Schreml S. 2012. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 26, 812–820.
Suematsu S., Matsusaka T., Matsuda T., Ohno S., Miyazaki J., Yamamura K., Hirano T., Kishimoto T. 1992. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 89, 232–235.
Ebisui C., Tsujinaka T., Morimoto T., Kan K., Iijima S., Yano M., Kominami E., Tanaka K., Monden M. 1995. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clin. Sci. (Lond.). 89, 431–439.
Tsujinaka T., Ebisui C., Fujita J., Kishibuchi M., Morimoto T., Ogawa A., Katsume A., Ohsugi Y., Kominami E., Monden M. 1995. Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin-6 transgenic mouse. Biochem. Biophys. Res. Commun. 207, 168–174.
Kitamura H., Kawata H., Takahashi F., Higuchi Y., Furuichi T., Ohkawa H. 1995. Bone marrow neutrophilia and suppressed bone turnover in human interleukin-6 transgenic mice. A cellular relationship among hematopoietic cells, osteoblasts, and osteoclasts mediated by stromal cells in bone marrow. Am. J. Pathol. 147, 1682–1692.
Katsume A., Saito H., Yamada Y., Yorozu K., Ueda O., Akamatsu K., Nishimoto N., Kishimoto T., Yoshizaki K., Ohsugi Y. 2002. Anti-interleukin 6 (IL-6) receptor antibody suppresses Castleman’s disease like symptoms emerged in IL-6 transgenic mice. Cytokine. 20, 304–311.
Suematsu S., Matsuda T., Aozasa K., Akira S., Nakano N., Ohno S., Miyazaki J., Yamamura K., Hirano T., Kishimoto T. 1989. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 86, 7547–7551.
Fattori E., Lazzaro D., Musiani P., Modesti A., Alonzi T., Ciliberto G. 1995. IL-6 expression in neurons of transgenic mice causes reactive astrocytosis and increase in ramified microglial cells but no neuronal damage. Eur. J. Neurosci. 7, 2441–2449.
De Benedetti F., Alonzi T., Moretta A., Lazzaro D., Costa P., Poli V., Martini A., Ciliberto G., Fattori E. 1997. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J. Clin. Invest. 99, 643–650.
De Benedetti F., Meazza C., Oliveri M., Pignatti P., Vivarelli M., Alonzi T., Fattori E., Garrone S., Barreca A., Martini A. 2001. Effect of IL-6 on IGF binding protein-3: A study in IL-6 transgenic mice and in patients with systemic juvenile idiopathic arthritis. Endocrinology. 142, 4818–4826.
Fattori E., Della Rocca C., Costa P., Giorgio M., Dente B., Pozzi L., Ciliberto G. 1994. Development of progressive kidney damage and myeloma kidney in interleukin-6 transgenic mice. Blood. 83, 2570–2579.
Lieskovska J., Guo D., Derman E. 2002. IL-6-overexpression brings about growth impairment potentially through a GH receptor defect. Growth Horm. IGF Res. 12, 388–398.
DiCosmo B.F., Geba G.P., Picarella D., Elias J.A., Rankin J.A., Stripp B.R., Whitsett J.A., Flavell R.A. 1994. Airway epithelial cell expression of Interleukin-6 in transgenic mice. Uncoupling of airway inflammation and bronchial hyperreactivity. J. Clin. Invest. 94, 2028–2035.
Kuhn C., 3rd, Homer R.J., Zhu Z., Ward N., Flavell R.A., Geba G.P., Elias J.A. 2000. Airway hyperresponsiveness and airway obstruction in transgenic mice. Morphologic correlates in mice overexpressing interleukin (IL)-11 and IL-6 in the lung. Am. J. Respir. Cell Mol. Biol. 22, 289–295.
Zvartsev R.V., Korshunova D.S., Gorshkova E.A., Nosenko M.A., Korneev K.V., Maksimenko O.G., Korobko I.V., Kuprash D.V., Drutskaya M.S., Nedospasov S.A., Deikin A.V. 2018. Neonatal lethality and inflammatory phenotype of the new transgenic mice with overexpression of human interleukin-6 in myeloid cells. Dokl. Biochem. Biophys. 483, 344–347.
Campbell I.L., Abraham C.R., Masliah E., Kemper P., Inglis J.D., Oldstone M.B., Mucke L. 1993. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl. Acad. Sci. U. S. A. 90, 10061–10065.
Turksen K., Kupper T., Degenstein L., Williams I., Fuchs E. 1992. Interleukin 6: Insights to its function in skin by overexpression in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 89, 5068–5072.
Lieskovska J., Guo D., Derman E. 2003. Growth impairment in IL-6-overexpressing transgenic mice is associated with induction of SOCS3 mRNA. Growth Horm. IGF Res. 13, 26–35.
De Benedetti F., Rucci N., Del Fattore A., Peruzzi B., Paro R., Longo M., Vivarelli M., Muratori F., Berni S., Ballanti P., Ferrari S., Teti A. 2006. Impaired skeletal development in interleukin-6-transgenic mice: A model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. 54, 3551–3563.
Korneev K.V., Sviryaeva E.N., Drutskaya M.S., Kuprash D.V., Nedospasov S.A. 2016. Development of a system for inducing Cre-dependent human interleukin-6 production in mouse and human cells. Ross. Immunol. Zh. 10, 188–192.
Drutskaya M.S., Gogoleva V.S., Atretkhany K.-S.N., Gubernatorova E.O., Zvartsev R.V., Nosenko M.A., Nedospasov S.A. 2018. Proinflammatory and immunoregulatory functions of interleukin 6 as identified by reverse genetics. Mol. Biol. (Moscow). 52 (6), 836–845.
Gorshkova E.A., Zvartsev R.V., Nosenko M.A., Korneev K.V., Kuprash D.V., Drutskaya M.S., Deikin A.V., Nedospasov S.A. 2018. A transgenic mouse with human interleukin-6 overexpression in myeloid cells. In Abstr. V Mezhd. konf. “POSTGENOM’2018. V poiskakh modelei personalizirovannoi meditsiny (POSTGENOME’2018: In Search of Personalized Medicine Models, Abstr. 5th Int. Conf.). Kazan: Kazan. Gos. Univ., p. 269.
Zeilhofer H.U., Schorr W. 2000. Role of interleukin-8 in neutrophil signaling. Curr. Opin. Hematol. 7, 178–182.
Elliott M.J., Finn A.H. 1993. Interaction between neutrophils and endothelium. Ann. Thorac. Surg. 56, 1503–1508.
Rot A. 1991. Chemotactic potency of recombinant human neutrophil attractant/activation protein-1 (interleukin-8) for polymorphonuclear leukocytes of different species. Cytokine. 3, 21–27.
Simonet W.S., Hughes T.M., Nguyen H.Q., Trebasky L.D., Danilenko D.M., Medlock E.S. 1994. Long-term impaired neutrophil migration in mice overexpressing human interleukin-8. J. Clin. Invest. 94, 1310–1319.
Kim M.R., Manoukian R., Yeh R., Silbiger S.M., Danilenko D.M., Scully S., Sun J., DeRose M.L., Stolina M., Chang D., Van G.Y., Clarkin K., Nguyen H.Q., Yu Y.B., Jing S., et al. 2002. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 100, 2330–2340.
Park M.H., Yoon D.Y., Ban J.O., Kim D.H., Lee D.H., Song S., Kim Y., Han S.B., Lee H.P., Hong J.T. 2015. Decreased severity of collagen antibody and lipopolysaccharide-induced arthritis in human IL-32beta overexpressed transgenic mice. Oncotarget. 6, 38566–38577.
Yun H.M., Kim J.A., Hwang C.J., Jin P., Baek M.K., Lee J.M., Hong J.E., Lee S.M., Han S.B., Oh K.W., Choi D.Y., Yoon D.Y., Hong J.T. 2015. Neuroinflammatory and amyloidogenic activities of IL-32 beta in Alzheimer’s disease. Mol. Neurobiol. 52, 341–352.
Jung Y.Y., Katila N., Neupane S., Shadfar S., Ojha U., Bhurtel S., Srivastav S., Son D.J., Park P.H., Yoon D.Y., Hong J.T., Choi D.Y. 2017. Enhanced dopaminergic neurotoxicity mediated by MPTP in IL-32 beta transgenic mice. Neurochem. Int. 102, 79–88.
Haak S., Croxford A.L., Kreymborg K., Heppner F.L., Pouly S., Becher B., Waisman A. 2009. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Invest. 119, 61–69.
Croxford A.L., Karbach S., Kurschus F.C., Wortge S., Nikolaev A., Yogev N., Klebow S., Schuler R., Reissig S., Piotrowski C., Brylla E., Bechmann I., Scheller J., Rose-John S., Thomas Wunderlich F., et al. 2014. IL‑6 regulates neutrophil microabscess formation in IL-17A-driven psoriasiform lesions. J. Invest. Dermatol. 134, 728–735.
Karbach S., Croxford A.L., Oelze M., Schuler R., Minwegen D., Wegner J., Koukes L., Yogev N., Nikolaev A., Reissig S., Ullmann A., Knorr M., Waldner M., Neurath M.F., Li H., et al. 2014. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler. Thromb. Vasc. Biol. 34, 2658–2668.
Kim S.H., Han S.Y., Azam T., Yoon D.Y., Dinarello C.A. 2005. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 22, 131–142.
Nold M.F., Nold-Petry C.A., Pott G.B., Zepp J.A., Saavedra M.T., Kim S.H., Dinarello C.A. 2008. Endogenous IL-32 controls cytokine and HIV-1 production. J. Immunol. 181, 557–565.
Li W., Yang F., Liu Y., Gong R., Liu L., Feng Y., Hu P., Sun W., Hao Q., Kang L., Wu J., Zhu Y. 2009. Negative feedback regulation of IL-32 production by iNOS activation in response to dsRNA or influenza virus infection. Eur. J. Immunol. 39, 1019–1024.
Bai X., Ovrutsky A.R., Kartalija M., Chmura K., Kamali A., Honda J.R., Oberley-Deegan R.E., Dinarello C.A., Crapo J.D., Chang L.Y., Chan E.D. 2011. IL-32 expression in the airway epithelial cells of patients with Mycobacterium avium complex lung disease. Int. Immunol. 23, 679–691.
Heinhuis B., Koenders M.I., van de Loo F.A., Netea M.G., van den Berg W.B., Joosten L.A. 2011. Inflammation-dependent secretion and splicing of IL-32 gamma in rheumatoid arthritis. Proc. Natl. Acad. Sci. U. S. A. 108, 4962–4967.
Zhang S., Guo G.L., Yang L.L., Sun L.Q. 2017. Elevated serum levels of ghrelin and TNF-alpha in patients with cyanotic and acyanotic congenital heart disease. World J. Pediatr. 13, 122–128.
Jiang X.G., Yang X.D., Lv Z., Zhuang P.H. 2018. Elevated serum levels of TNF-alpha, IL-8, and ECP can be involved in the development and progression of bronchial asthma. J. Asthma. 55, 111–118.
Ciebiera M., Wlodarczyk M., Wrzosek M., Wojtyla C., Blazej M., Nowicka G., Lukaszuk K., Jakiel G. 2018. TNF-alpha serum levels are elevated in women with clinically symptomatic uterine fibroids. Int. J. Immunopathol. Pharmacol. 32, 2058738418779461.
Chen Y.L., Qiao Y.C., Xu Y., Ling W., Pan Y.H., Huang Y.C., Geng L.J., Zhao H.L., Zhang X.X. 2017. Serum TNF-alpha concentrations in type 2 diabetes mellitus patients and diabetic nephropathy patients: A systematic review and meta-analysis. Immunol. Lett. 186, 52–58.
Luo Y., He H., Zhang M., Huang X., Fan N. 2016. Altered serum levels of TNF-alpha, IL-6 and IL-18 in manic, depressive, mixed state of bipolar disorder patients. Psychiatry Res. 244, 19–23.
Ozler K., Aktas E., Atay C., Yilmaz B., Arikan M., Gungor S. 2016. Serum and knee synovial fluid matrixmetalloproteinase-13 and tumor necrosis factor-alpha levels in patients with late stage osteoarthritis. Acta Orthop. Traumatol. Turc. 50, 670–673.
Georgopoulos S., Plows D., Kollias G. 1996. Transmembrane TNF is sufficient to induce localized tissue toxicity and chronic inflammatory arthritis in transgenic mice. J. Inflamm. 46, 86–97.
Kontoyiannis D., Pasparakis M., Pizarro T.T., Cominelli F., Kollias G. 1999. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: Implications for joint and gut-associated immunopathologies. Immunity. 10, 387–398.
Lewis M., Tartaglia L.A., Lee A., Bennett G.L., Rice G.C., Wong G.H., Chen E.Y., Goeddel D.V. 1991. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc. Natl. Acad. Sci. U. S. A. 88, 2830–2834.
Atretkhany K.N., Mufazalov I.A., Dunst J., Kuchmiy A., Gogoleva V.S., Andruszewski D., Drutskaya M.S., Faustman D.L., Schwabenland M., Prinz M., Kruglov A.A., Waisman A., Nedospasov S.A. 2018. Intrinsic TNFR2 signaling in T regulatory cells provides protection in CNS autoimmunity. Proc. Natl. Acad. Sci. U. S. A. 115, 13051–13056.
Li P., Schwarz E.M. 2003. The TNF-alpha transgenic mouse model of inflammatory arthritis. Springer Semin. Immunopathol. 25, 19–33.
Horta-Baas G., Romero-Figueroa M.D.S., Montiel-Jarquin A.J., Pizano-Zarate M.L., Garcia-Mena J., Ramirez-Duran N. 2017. Intestinal dysbiosis and rheumatoid arthritis: A link between gut microbiota and the pathogenesis of rheumatoid arthritis. J. Immunol. Res. 2017, 4835189.
Maeda Y., Takeda K. 2017. Role of gut microbiota in rheumatoid arthritis. J. Clin. Med. 6.
Ruddle N.H., Bergman C.M., McGrath K.M., Lingenheld E.G., Grunnet M.L., Padula S.J., Clark R.B. 1990. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J. Exp. Med. 172, 1193–1200.
Xanthoulea S., Gijbels M.J., van der Made I., Mujcic H., Thelen M., Vergouwe M.N., Ambagts M.H., Hofker M.H., de Winther M.P. 2008. P55 tumour necrosis factor receptor in bone marrow-derived cells promotes atherosclerosis development in low-density lipoprotein receptor knock-out mice. Cardiovasc. Res. 80, 309–318.
Jung I.H., Choi J.H., Jin J., Jeong S.J., Jeon S., Lim C., Lee M.R., Yoo J.Y., Sonn S.K., Kim Y.H., Choi B.K., Kwon B.S., Seoh J.Y., Lee C.W., Kim D.Y., Oh G.T. 2014. CD137-inducing factors from T cells and macrophages accelerate the destabilization of atherosclerotic plaques in hyperlipidemic mice. FASEB J. 28, 4779–4791.
Fujii S. 2015. Atherosclerosis, chronic inflammation, and thrombosis: in search of the missing link in laboratory medicine. Rinsho Byori. 63, 605–611.
Glosli H., Prydz H., Roald B. 2004. Involution of thymus and lymphoid depletion in mice expressing the hTNF transgene. APMIS. 112, 63–73.
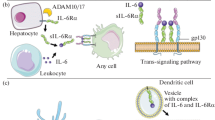
Rose-John S. 2012. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 8, 1237–1247.
Ueda O., Tateishi H., Higuchi Y., Fujii E., Kato A., Kawase Y., Wada N.A., Tachibe T., Kakefuda M., Goto C., Kawaharada M., Shimaoka S., Hattori K., Jishage K. 2013. Novel genetically-humanized mouse model established to evaluate efficacy of therapeutic agents to human interleukin-6 receptor. Sci. Rep. 3, 1196.
Sitenga J., Aird G., Ahmed A., Silberstein P.T. 2018. Impact of siltuximab on patient-related outcomes in multicentric Castleman’s disease. Patient Relat. Outcome Meas. 9, 35–41.
van Hamburg J.P., Tas S.W. 2018. Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis. J. Autoimmun. 87, 69–81.
Waisman A., Hauptmann J., Regen T. 2015. The role of IL-17 in CNS diseases. Acta Neuropathol. 129, 625–637.
Malakouti M., Brown G.E., Wang E., Koo J., Levin E.C. 2015. The role of IL-17 in psoriasis. J. Dermatolog. Treat. 26, 41–44.
Chen X., Zhao S., Tang X., Ge H., Liu P. 2011. Neutralization of mouse interleukin-17 bioactivity inhibits corneal allograft rejection. Mol. Vis. 17, 2148–2156.
Gorbacheva V., Fan R., Li X., Valujskikh A. 2010. Interleukin-17 promotes early allograft inflammation. Am. J. Pathol. 177, 1265–1273.
Schleich F.N., Chevremont A., Paulus V., Henket M., Manise M., Seidel L., Louis R. 2014. Importance of concomitant local and systemic eosinophilia in uncontrolled asthma. Eur. Respir. J. 44, 97–108.
Drago F., Cogorno L., Agnoletti A.F., Parodi A. 2015. Role of peripheral eosinophilia in adverse cutaneous drug reactions. Eur. Rev. Med. Pharmacol. Sci. 19, 2008–2009.
Mitre E. 2013. Eosinophilia: A diagnostic clue for nonbacterial diseases in patients with systemic inflammatory response syndrome. Crit. Care Med. 41, 2464–2465.
Senra L., Mylonas A., Kavanagh R.D., Fallon P.G., Conrad C., Borowczyk-Michalowska J., Wrobel L.J., Kaya G., Yawalkar N., Boehncke W.H., Brembilla N.C. 2019. IL-17E (IL-25) enhances innate immune responses during skin inflammation. J. Invest. Dermatol. pii: S0022-202X(19)30101-0. https://doi.org/10.1016/j.jid.2019.01.021
Mosolygo T., Spengler G., Endresz V., Laczi K., Perei K., Burian K. 2013. IL-17E production is elevated in the lungs of Balb/c mice in the later stages of Chlamydia muridarum infection and re-infection. In Vivo. 27, 787–792.
Senra L., Stalder R., Alvarez Martinez D., Chizzolini C., Boehncke W.H., Brembilla N.C. 2016. Keratinocyte-derived IL-17E contributes to inflammation in psoriasis. J. Invest. Dermatol. 136, 1970–1980.
Kang J.W., Choi S.C., Cho M.C., Kim H.J., Kim J.H., Lim J.S., Kim S.H., Han J.Y., Yoon D.Y. 2009. A proinflammatory cytokine interleukin-32 beta promotes the production of an anti-inflammatory cytokine interleukin-10. Immunology. 128, e532–e540.
ACKNOWLEDGMENTS
We are grateful to A.V. Deikin for constructing the hIL-6 Tg mouse strain at the Collective Access Center of the Institute of Gene Biology (Russian Academy of Sciences) and P.V. Matveev for help in figure preparation.
Funding
This work was supported by the Russian Science Foundation (project no. 19-75-30032).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by T. Tkacheva
Abbreviations: ApoE, apolipoprotein E; ARE, AU-rich element; Cas9, CRISPR-associated protein 9; CFU, colony-forming unit; CRISPR, clustered regularly interspaced short palindromic repeat; GM-CSF, granulocyte–macrophage colony-stimulating factor; EGFP, enhanced green fluorescent protein; IL, interleukin; IL-6R, IL-6 receptor; iNOS, induced NO synthase; M-CSF, macrophage colony-stimulating factor; Th2, T helper cell type 2; TNF, tumor necrosis factor; TNFR, TNF receptor; UTR, untranslated region; ESC, embryonic stem cell.
Rights and permissions
About this article
Cite this article
Gorshkova, E.A., Zvartsev, R.V., Drutskaya, M.S. et al. Humanized Mouse Models as a Tool to Study Proinflammatory Cytokine Overexpression. Mol Biol 53, 665–680 (2019). https://doi.org/10.1134/S0026893319050078
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893319050078