Abstract
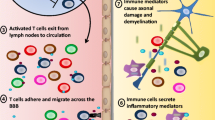
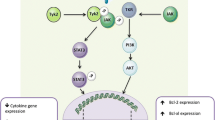
Cytokines of the IL-17 family are uniquely placed on the border between immune cells and tissue. Although IL-17 was originally found to induce the activation and mobilization of neutrophils to sites of inflammation, its tissue-specific function is not yet fully understood. The best-studied IL-17 family members, IL-17A and IL-17F, are both typically produced by immune cells such as Th17, γδ T cells and innate lymphoid cells group 3. However, the cells that respond to these cytokines are mostly found in inflamed tissue. As seen in psoriatic skin lesions or in joints of rheumatoid arthritis patients, high levels of IL-17 have been detected in the central nervous system (CNS) during inflammatory responses. Here, we provide a general review of the molecular function of IL-17 and its role in the CNS in particular. Of the different inflammatory conditions of the CNS, we found multiple sclerosis (MS) to be the one most associated with the presence of Th17 cells and IL-17. In particular, many studies using the murine model for MS, experimental autoimmune encephalomyelitis, found a clear association of Th17 and IL-17 with disease severity and progression. We summarize the recent advances made in correlating the presence of IL-17 with impaired blood–brain barrier integrity as well as the activation of astrocytes and microglia and the consequences for disease progression. There is also evidence that IL-17 plays a pathogenic role in the post-ischemic phase of stroke as well as its experimental model. We review the limited but promising data on the sources of post-stroke IL-17 production and its effects on CNS-resident target cells. In addition to MS and stroke, there is also evidence linking high levels of IL-17 to depression, as a frequent comorbidity of several inflammatory diseases, as well as to different types of infections of the CNS. The evidence we supply here suggests that inhibiting the function of the IL-17 cytokine family could have a beneficial effect on pathogenic conditions in the CNS.


Similar content being viewed by others
References
Bajpai A, Prasad KN, Mishra P, Singh AK, Gupta RK, Ojha BK (2014) Distinct cytokine pattern in response to different bacterial pathogens in human brain abscess. J Neuroimmunol 273:96–102. doi:10.1016/j.jneuroim.2014.05.009
Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479:538–541. doi:10.1038/nature10554
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238. doi:10.1038/nature04753
Beurel E, Harrington LE, Jope RS (2013) Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry 73:622–630. doi:10.1016/j.biopsych.2012.09.021
Brait VH, Arumugam TV, Drummond GR, Sobey CG (2012) Importance of T lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metab 32:598–611. doi:10.1038/jcbfm.2012.6
Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, Sireci G, Fournie JJ, Dieli F (2011) Differentiation, phenotype, and function of interleukin-17-producing human Vgamma9Vdelta2 T cells. Blood 118:129–138. doi:10.1182/blood-2011-01-331298
Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L (1993) Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci 90:10061–10065
Carden DL, Granger DN (2000) Pathophysiology of ischaemia-reperfusion injury. J Pathol 190:255–266. doi:10.1002/(SICI)1096-9896(200002)190:3<255:AID-PATH526>3.0.CO;2-6
Chang SH, Reynolds JM, Pappu BP, Chen G, Martinez GJ, Dong C (2011) Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity 35:611–621. doi:10.1016/j.immuni.2011.09.010
Chisholm SP, Cervi AL, Nagpal S, Lomax AE (2012) Interleukin-17A increases neurite outgrowth from adult postganglionic sympathetic neurons. J Neurosci 32:1146–1155. doi:10.1523/JNEUROSCI.5343-11.2012
Conti HR, Peterson AC, Brane L, Huppler AR, Hernandez-Santos N, Whibley N, Garg AV, Simpson-Abelson MR, Gibson GA, Mamo AJ et al (2014) Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med 211:2075–2084. doi:10.1084/jem.20130877
Croxford AL, Karbach S, Kurschus FC, Wortge S, Nikolaev A, Yogev N, Klebow S, Schuler R, Reissig S, Piotrowski C et al (2014) IL-6 regulates neutrophil microabscess formation in IL-17A-driven psoriasiform lesions. J Invest Dermatol 134:728–735. doi:10.1038/jid.2013.404
Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T et al (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744–748. doi:10.1038/nature01355
Das Sarma J, Ciric B, Marek R, Sadhukhan S, Caruso ML, Shafagh J, Fitzgerald DC, Shindler KS, Rostami A (2009) Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J Neuroinflamm 6:14. doi:10.1186/1742-2094-6-14
de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Janniere L et al (2008) Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med 205:1543–1550. doi:10.1084/jem.20080321
Dong C (2008) Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol Rev 226:80–86. doi:10.1111/j.1600-065X.2008.00709.x
Elain G, Jeanneau K, Rutkowska A, Mir AK, Dev KK (2014) The selective anti-IL17A monoclonal antibody secukinumab (AIN457) attenuates IL17A-induced levels of IL6 in human astrocytes. Glia 62:725–735. doi:10.1002/glia.22637
Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O’Connor W Jr, Rongvaux A, Van Rooijen N, Haberman AM et al (2011) Control of TH17 cells occurs in the small intestine. Nature 475:514–518. doi:10.1038/nature10228
Gaffen SL (2011) Recent advances in the IL-17 cytokine family. Curr Opin Immunol 23:613–619. doi:10.1016/j.coi.2011.07.006
Gaffen SL (2009) Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9:556–567. doi:10.1038/nri2586
Gaffen SL, Jain R, Garg AV, Cua DJ (2014) The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 14:585–600. doi:10.1038/nri3707
Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E et al (2009) Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40:1849–1857. doi:10.1161/STROKEAHA.108.534503
Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV, Leypoldt F, Simova O et al (2012) Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 120:3793–3802. doi:10.1182/blood-2012-02-412726
Girolomoni G, Mrowietz U, Paul C (2012) Psoriasis: rationale for targeting interleukin-17. Br J Dermatol 167:717–724. doi:10.1111/j.1365-2133.2012.11099.x
Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A (2009) IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest 119:61–69. doi:10.1172/JCI35997
Haas JD, Ravens S, Duber S, Sandrock I, Oberdorfer L, Kashani E, Chennupati V, Fohse L, Naumann R, Weiss S et al (2012) Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity 37:48–59. doi:10.1016/j.immuni.2012.06.003
Han QQ, Yu J (2014) Inflammation: a mechanism of depression? Neurosci Bull 30:515–523. doi:10.1007/s12264-013-1439-3
Hayakawa K, Qiu J, Lo EH (2010) Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann N Y Acad Sci 1207:50–57. doi:10.1111/j.1749-6632.2010.05728.x
He JJ, Li S, Shu HF, Yu SX, Liu SY, Yin Q, Yang H (2013) The interleukin 17 system in cortical lesions in focal cortical dysplasias. J Neuropathol Exp Neurol 72:152–163. doi:10.1097/NEN.0b013e318281262e
Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R (2005) Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol 237:123–130. doi:10.1016/j.cellimm.2005.11.002
Holley MM, Kielian T (2012) Th1 and Th17 cells regulate innate immune responses and bacterial clearance during central nervous system infection. J Immunol 188:1360–1370. doi:10.4049/jimmunol.1101660
Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A et al (2010) Cellular mechanisms of IL-17-induced blood–brain barrier disruption. Faseb J 24:1023–1034. doi:10.1096/fj.09-141978
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121–1133. doi:10.1016/j.cell.2006.07.035
Iwakura Y, Ishigame H, Saijo S, Nakae S (2011) Functional specialization of interleukin-17 family members. Immunity 34:149–162. doi:10.1016/j.immuni.2011.02.012
Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C et al (2010) Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 32:414–425. doi:10.1016/j.immuni.2010.03.004
Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao J, Chandrasekharan U, DiCorleto PE, Trapp BD, Ransohoff RM et al (2013) Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat Neurosci 16:1401–1408. doi:10.1038/nn.3505
Karbach S, Croxford AL, Oelze M, Schuler R, Minwegen D, Wegner J, Koukes L, Yogev N, Nikolaev A, Reissig S et al (2014) Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol 34:2658–2668. doi:10.1161/ATVBAHA.114.304108
Kawanokuchi J, Shimizu K, Nitta A, Yamada K, Mizuno T, Takeuchi H, Suzumura A (2008) Production and functions of IL-17 in microglia. J Neuroimmunol 194:54–61. doi:10.1016/j.jneuroim.2007.11.006
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A (2007) Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med 13:1173–1175. doi:10.1038/nm1651
Kielian T, Haney A, Mayes PM, Garg S, Esen N (2005) Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect Immun 73:7428–7435. doi:10.1128/IAI.73.11.7428-7435.2005
Kim JW, Kim YK, Hwang JA, Yoon HK, Ko YH, Han C, Lee HJ, Ham BJ, Lee HS (2013) Plasma levels of IL-23 and IL-17 before and after antidepressant treatment in patients with major depressive disorder. Psychiatry Investig 10:294–299. doi:10.4306/pi.2013.10.3.294
Kira J, Yamasaki K, Horiuchi I, Ohyagi Y, Taniwaki T, Kawano Y (1999) Changes in the clinical phenotypes of multiple sclerosis during the past 50 years in Japan. J Neurol Sci 166:53–57
Kleinschek MA, Muller U, Brodie SJ, Stenzel W, Kohler G, Blumenschein WM, Straubinger RK, McClanahan T, Kastelein RA, Alber G (2006) IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol 176:1098–1106
Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G (2010) Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 115:3835–3842. doi:10.1182/blood-2009-10-249078
Kolls JK, Linden A (2004) Interleukin-17 family members and inflammation. Immunity 21:467–476. doi:10.1016/j.immuni.2004.08.018
Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y (2006) IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 177:566–573
Kostic M, Dzopalic T, Zivanovic S, Zivkovic N, Cvetanovic A, Stojanovic I, Vojinovic S, Marjanovic G, Savic V, Colic M (2014) IL-17 and glutamate excitotoxicity in the pathogenesis of multiple sclerosis. Scand J Immunol 79:181–186. doi:10.1111/sji.12147
Kostulas N, Pelidou SH, Kivisakk P, Kostulas V, Link H (1999) Increased IL-1beta, IL-8, and IL-17 mRNA expression in blood mononuclear cells observed in a prospective ischemic stroke study. Stroke 30:2174–2179
Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM (2010) The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 146:891–895. doi:10.1001/archdermatol.2010.186
Lassmann H (2014) Mechanisms of white matter damage in multiple sclerosis. Glia 62:1816–1830. doi:10.1002/glia.22597
Lee YK, Menezes JS, Umesaki Y, Mazmanian SK (2011) Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci 108(Suppl 1):4615–4622. doi:10.1073/pnas.1000082107
Li GZ, Zhong D, Yang LM, Sun B, Zhong ZH, Yin YH, Cheng J, Yan BB, Li HL (2005) Expression of interleukin-17 in ischemic brain tissue. Scand J Immunol 62:481–486. doi:10.1111/j.1365-3083.2005.01683.x
Li HL, Kostulas N, Huang YM, Xiao BG, van der Meide P, Kostulas V, Giedraitas V, Link H (2001) IL-17 and IFN-gamma mRNA expression is increased in the brain and systemically after permanent middle cerebral artery occlusion in the rat. J Neuroimmunol 116:5–14
Li Z, Li K, Zhu L, Kan Q, Yan Y, Kumar P, Xu H, Rostami A, Zhang GX (2013) Inhibitory effect of IL-17 on neural stem cell proliferation and neural cell differentiation. BMC Immunol 14:20. doi:10.1186/1471-2172-14-20
Liu G, Guo J, Liu J, Wang Z, Liang D (2014) Toll-like receptor signaling directly increases functional IL-17RA expression in neuroglial cells. Clin Immunol 154:127–140. doi:10.1016/j.clim.2014.07.006
Liu X, He F, Pang R, Zhao D, Qiu W, Shan K, Zhang J, Lu Y, Li Y, Wang Y (2014) Interleukin-17 (IL-17)-induced microRNA 873 (miR-873) contributes to the pathogenesis of experimental autoimmune encephalomyelitis by targeting A20 ubiquitin-editing enzyme. J Biol Chem 289:28971–28986. doi:10.1074/jbc.M114.577429
Liu Y, Ho RC, Mak A (2012) The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis 15:183–187. doi:10.1111/j.1756-185X.2011.01673.x
Lubberts E (2003) The role of IL-17 and family members in the pathogenesis of arthritis. Curr Opin Investig Drugs 4:572–577
Lv M, Liu Y, Zhang J, Sun L, Liu Z, Zhang S, Wang B, Su D, Su Z (2011) Roles of inflammation response in microglia cell through Toll-like receptors 2/interleukin-23/interleukin-17 pathway in cerebral ischemia/reperfusion injury. Neuroscience 176:162–172. doi:10.1016/j.neuroscience.2010.11.066
Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC (2008) Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med 205:1551–1557. doi:10.1084/jem.20080218
Mardiguian S, Serres S, Ladds E, Campbell SJ, Wilainam P, McFadyen C, McAteer M, Choudhury RP, Smith P, Saunders F et al (2013) Anti-IL-17A treatment reduces clinical score and VCAM-1 expression detected by in vivo magnetic resonance imaging in chronic relapsing EAE ABH mice. Am J Pathol 182:2071–2081. doi:10.1016/j.ajpath.2013.02.029
Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, Craft J (2009) Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol 10:1125–1132. doi:10.1038/ni.1783
Meares GP, Ma X, Qin H, Benveniste EN (2012) Regulation of CCL20 expression in astrocytes by IL-6 and IL-17. Glia 60:771–781. doi:10.1002/glia.22307
Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, van Noort JM (2003) Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia 43:243–253. doi:10.1002/glia.10259
Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML et al (2008) Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452:773–776. doi:10.1038/nature06764
Murphy AC, Lalor SJ, Lynch MA, Mills KH (2010) Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun 24:641–651. doi:10.1016/j.bbi.2010.01.014
Nichols JR, Aldrich AL, Mariani MM, Vidlak D, Esen N, Kielian T (2009) TLR2 deficiency leads to increased Th17 infiltrates in experimental brain abscesses. J Immunol 182:7119–7130. doi:10.4049/jimmunol.0802656
Paintlia MK, Paintlia AS, Singh AK, Singh I (2011) Synergistic activity of interleukin-17 and tumor necrosis factor-alpha enhances oxidative stress-mediated oligodendrocyte apoptosis. J Neurochem 116:508–521. doi:10.1111/j.1471-4159.2010.07136.x
Pappu R, Ramirez-Carrozzi V, Sambandam A (2011) The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology 134:8–16. doi:10.1111/j.1365-2567.2011.03465.x
Peerschke EI, Yin W, Ghebrehiwet B (2010) Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol 47:2170–2175. doi:10.1016/j.molimm.2010.05.009
Pinsky DJ, Naka Y, Liao H, Oz MC, Wagner DD, Mayadas TN, Johnson RC, Hynes RO, Heath M, Lawson CA et al (1996) Hypoxia-induced exocytosis of endothelial cell Weibel-Palade bodies. A mechanism for rapid neutrophil recruitment after cardiac preservation. J Clin Invest 97:493–500. doi:10.1172/JCI118440
Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J et al (2007) The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 8:247–256. doi:10.1038/ni1439
Ransohoff RM (2005) Natalizumab and PML. Nat Neurosci 8:1275. doi:10.1038/nn1005-1275
Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F (2009) C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10:514–523. doi:10.1038/ni.1716
Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, Sekiya T, Ooboshi H, Kitazono T, Yanagawa T et al (2012) Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med 18:911–917. doi:10.1038/nm.2749
Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ et al (2009) Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med 15:946–950. doi:10.1038/nm.1999
Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Luenstedt S, Rinnenthal JL et al (2010) In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity 33:424–436. doi:10.1016/j.immuni.2010.08.018
Sonobe Y, Liang J, Jin S, Zhang G, Takeuchi H, Mizuno T, Suzumura A (2008) Microglia express a functional receptor for interleukin-23. Biochem Biophys Res Commun 370:129–133. doi:10.1016/j.bbrc.2008.03.059
Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D et al (2006) Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 7:937–945. doi:10.1038/ni1376
Sutton CE, Mielke LA, Mills KH (2012) IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol 42:2221–2231. doi:10.1002/eji.201242569
Tanaka S, Yoshimoto T, Naka T, Nakae S, Iwakura Y, Cua D, Kubo M (2009) Natural occurring IL-17 producing T cells regulate the initial phase of neutrophil mediated airway responses. J Immunol 183:7523–7530. doi:10.4049/jimmunol.0803828
Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L (2008) Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172:146–155. doi:10.2353/ajpath.2008.070690
van den Berg WB, Miossec P (2009) IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol 5:549–553. doi:10.1038/nrrheum.2009.179
Vidlak D, Kielian T (2012) Differential effects of interleukin-17 receptor signaling on innate and adaptive immunity during central nervous system bacterial infection. J Neuroinflamm 9:128. doi:10.1186/1742-2094-9-128
Waisman A (2012) To be 17 again–anti-interleukin-17 treatment for psoriasis. N Engl J Med 366:1251–1252. doi:10.1056/NEJMe1201071
Wang DD, Zhao YF, Wang GY, Sun B, Kong QF, Zhao K, Zhang Y, Wang JH, Liu YM, Mu LL et al (2009) IL-17 potentiates neuronal injury induced by oxygen-glucose deprivation and affects neuronal IL-17 receptor expression. J Neuroimmunol 212:17–25. doi:10.1016/j.jneuroim.2009.04.007
Wang X, Deckert M, Xuan NT, Nishanth G, Just S, Waisman A, Naumann M, Schluter D (2013) Astrocytic A20 ameliorates experimental autoimmune encephalomyelitis by inhibiting NF-kappaB- and STAT1-dependent chemokine production in astrocytes. Acta Neuropathol. doi:10.1007/s00401-013-1183-9
Weaver CT, Hatton RD, Mangan PR, Harrington LE (2007) IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25:821–852. doi:10.1146/annurev.immunol.25.022106.141557
Wieghofer P, Knobeloch KP, Prinz M (2015) Genetic targeting of microglia. Glia 63:1–22. doi:10.1002/glia.22727
Xiao Y, Jin J, Chang M, Nakaya M, Hu H, Zou Q, Zhou X, Brittain GC, Cheng X, Sun SC (2014) TPL2 mediates autoimmune inflammation through activation of the TAK1 axis of IL-17 signaling. J Exp Med 211:1689–1702. doi:10.1084/jem.20132640
Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC et al (2014) Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 211:1533–1549. doi:10.1084/jem.20132477
Yan Y, Ding X, Li K, Ciric B, Wu S, Xu H, Gran B, Rostami A, Zhang GX (2012) CNS-specific therapy for ongoing EAE by silencing IL-17 pathway in astrocytes. Mol Ther 20:1338–1348. doi:10.1038/mt.2012.12
Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK (1995) Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3:811–821
Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ (1995) Human IL-17: a novel cytokine derived from T cells. J Immunol 155:5483–5486
Yi H, Bai Y, Zhu X, Lin L, Zhao L, Wu X, Buch S, Wang L, Chao J, Yao H (2014) IL-17A induces MIP-1alpha expression in primary astrocytes via Src/MAPK/PI3K/NF-kB pathways: implications for multiple sclerosis. J Neuroimmune Pharmacol 9:629–641. doi:10.1007/s11481-014-9553-1
Yilmaz G, Arumugam TV, Stokes KY, Granger DN (2006) Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 113:2105–2112. doi:10.1161/CIRCULATIONAHA.105.593046
Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A et al (2013) Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38:79–91. doi:10.1016/j.immuni.2012.12.001
Zepp J, Wu L, Li X (2011) IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol 32:232–239. doi:10.1016/j.it.2011.02.007
Zhang J, Mao X, Zhou T, Cheng X, Lin Y (2014) IL-17A contributes to brain ischemia reperfusion injury through calpain-TRPC6 pathway in mice. Neuroscience 274:419–428. doi:10.1016/j.neuroscience.2014.06.001
Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Maruo T, Date I, Yoshino T, Ohtsuka A, Mori S et al (2011) Anti-high mobility group box-1 monoclonal antibody protects the blood–brain barrier from ischemia-induced disruption in rats. Stroke 42:1420–1428. doi:10.1161/STROKEAHA.110.598334
Zimmermann J, Krauthausen M, Hofer MJ, Heneka MT, Campbell IL, Muller M (2013) CNS-targeted production of IL-17A induces glial activation, microvascular pathology and enhances the neuroinflammatory response to systemic endotoxemia. PLoS One 8:e57307. doi:10.1371/journal.pone.0057307
Acknowledgments
The authors are thankful to Christie Dietz for careful proofreading of the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) Research Unit (FOR) 1336 and the SFB/TRR128 to AW and in addition by the EU ITN project Neurokine to AW. AW is a member of the Research Center for Immunotherapy (FZI) and the Focal program in Translational Neuroscience (FTN) of the Johannes Gutenberg University of Mainz.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waisman, A., Hauptmann, J. & Regen, T. The role of IL-17 in CNS diseases. Acta Neuropathol 129, 625–637 (2015). https://doi.org/10.1007/s00401-015-1402-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1402-7