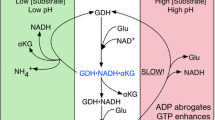
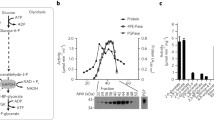
Abstract
2-Oxo acid dehydrogenase complexes are important metabolic checkpoints functioning at the intercept of sugar and amino acid degradation. This review presents a short summary of architectural, catalytic, and regulatory principles of the complexes structure and function, based on recent advances in studies of well-characterized family members. Special attention is given to use of synthetic phosphonate and phosphinate analogs of 2-oxo acids as selective and efficient inhibitors of the cognate complexes in biological systems of bacterial, plant, and animal origin. We summarize our own results concerning the application of synthetic analogs of 2-oxo acids in situ and in vivo to reveal functional interactions between 2-oxo acid dehydrogenase complexes and other components of metabolic networks specific to different cells and tissues. Based on our study of glutamate excitotoxicity in cultured neurons, we show how a modulation of metabolism by specific inhibition of its key reaction may be employed to correct pathologies. This approach is further developed in our study on the action of the phosphonate analog of 2-oxoglutarate in animals. The study revealed that upregulation of 2-oxoglutarate dehydrogenase complex is involved in animal stress response and may provide increased resistance to damaging effects, underlying so-called preconditioning. The presented analysis of published data suggests synthetic inhibitors of metabolic checkpoints as promising tools to solve modern challenges of systems biology, metabolic engineering, and medicine.
Similar content being viewed by others
Abbreviations
- AcP:
-
acetyl phosphonate
- AcPH:
-
acetyl phosphinate
- AcPMe:
-
methyl ester of acetyl phosphonate
- AcPMe2 :
-
dimethyl ester of acetyl phosphonate
- BCDHC:
-
branched-chain 2-oxo acid dehydrogenase complex
- BDK:
-
branched-chain 2-oxo acid dehydrogenase kinase
- BDP:
-
branched-chain 2-oxo acid dehydrogenase phosphatase
- DHTKD1:
-
dehydrogenase and transketolase domain containing protein 1
- MeAcPH:
-
methyl (acetyl) phosphinate
- OGDH:
-
2-oxoglutarate dehydrogenase
- OGDHC:
-
2-oxoglutarate dehydrogenase complex
- OGDHL:
-
2-oxoglutarate dehydrogenase-like protein
- PDHC:
-
pyruvate dehydrogenase complex
- PDK:
-
pyruvate dehydrogenase kinase
- PDP:
-
pyruvate dehydrogenase phosphatase
- PESP:
-
phosphonoethyl ester of succinyl phosphonate
- PMSP:
-
phosphonomethyl ester of succinyl phosphonate
- SP:
-
succinyl phosphonate
- ThDP:
-
thiamine diphosphate
References
Bunik, V. I., Raddatz, G., and Strumilo, S. A. (2013) Translating enzymology into metabolic regulation: the case of the 2-oxoglutarate dehydrogenase multienzyme complex, Curr. Chem. Biol., 7, 74–93.
Bunik, V. I., Tylicki, A., and Lukashev, N. V. (2013) Thiamin diphosphate-dependent enzymes: from enzymology to metabolic regulation, drug design and disease models, FEBS J., 280, 6412–6442.
Dahl, H. H., Brown, R. M., Hutchison, W. M., Maragos, C., and Brown, G. K. (1990) A testis-specific form of the human pyruvate dehydrogenase E1 alpha subunit is coded for by an intronless gene on chromosome 4, Genomics, 8, 225–232.
Bunik, V. I., and Degtyarev, D. (2008) Structure–function relationships in the 2-oxo acid dehydrogenase family: substrate-specific signatures and functional predictions for the 2-oxoglutarate dehydrogenase-like proteins, Proteins, 71, 874–890.
Bunik, V., Kaehne, T., Degtyarev, D., Shcherbakova, T., and Reiser, G. (2008) Novel isoenzyme of 2-oxoglutarate dehydrogenase is identified in brain, but not in heart, FEBS J., 275, 4990–5006.
Denton, R. M., Pullen, T. J., Armstrong, C. T., Heesom, K. J., and Rutter, G. A. (2016) Calcium-insensitive splice variants of mammalian E1 subunit of 2-oxoglutarate dehydrogenase complex with tissue-specific patterns of expression, Biochem. J., 473, 1165–1178.
Hoque, M. O., Kim, M. S., Ostrow, K. L., Liu, J., Wisman, G. B., Park, H. L., Poeta, M. L., Jeronimo, C., Henrique, R., Lendvai, A., Schuuring, E., Begum, S., Rosenbaum, E., Ongenaert, M., Yamashita, K., Califano, J., Westra, W., Van der Zee, A. G., Van Criekinge, W., and Sidransky, D. (2008) Genome-wide promoter analysis uncovers portions of the cancer methylome, Cancer Res., 68, 2661–2670.
Ostrow, K. L., Park, H. L., Hoque, M. O., Kim, M. S., Liu, J., Argani, P., Westra, W., Van Criekinge, W., and Sidransky, D. (2009) Pharmacologic unmasking of epigenetically silenced genes in breast cancer, Clin. Cancer Res., 15, 1184–1191.
Fedorova, M. S., Kudryavtseva, A. V., Lakunina, V. A., Snezhlina, A. V., Volchenko, N. N., Slavnova, E. N., Danilova, T. V., Sadritdinova, A. F., Melnikova, N. V., Belova, A. A., Klimina, K. M., Sidorov, D. V., Alexeev, B. Y., Kaprin, A. D., Dmitriev, A. A., and Krasnov, G. S. (2015) Downregulation of OGDHL expression is associated with promoter hypermethylation in colorectal cancer, Mol. Biol. (Moscow), 49, 678–688.
Sen, T., Sen, N., Noordhuis, M. G., Ravi, R., Wu, T. C., Ha, P. K., Sidransky, D., and Hoque, M. O. (2012) OGDHL is a modifier of AKT-dependent signaling and NF-kappaB function, PLoS One, 7, e48770.
Danhauser, K., Sauer, S. W., Haack, T. B., Wieland, T., Staufner, C., Graf, E., Zschocke, J., Strom, T. M., Traub, T., Okun, J. G., Meitinger, T., Hoffmann, G. F., Prokisch, H., and Kolker, S. (2012) DHTKD1 mutations cause 2aminoadipic and 2-oxoadipic aciduria, Am. J. Hum. Genet., 91, 1082–1087.
Hagen, J., te Brinke, H., Wanders, R. J., Knegt, A. C., Oussoren, E., Hoogeboom, A. J., Ruijter, G. J., Becker, D., Schwab, K. O., Franke, I., Duran, M., Waterham, H. R., Sass, J. O., and Houten, S. M. (2015) Genetic basis of alpha-aminoadipic and alpha-ketoadipic aciduria, J. Inherit. Metab. Dis., 38, 873–879.
Stiles, A. R., Venturoni, L., Mucci, G., Elbalalesy, N., Woontner, M., Goodman, S., and Abdenur, J. E. (2015) New cases of DHTKD1 mutations in patients with 2ketoadipic aciduria, JIMD Rep., 25, 15–19.
Bunik, V., Westphal, A. H., and De Kok, A. (2000) Kinetic properties of the 2-oxoglutarate dehydrogenase complex from Azotobacter vinelandii evidence for the formation of a precatalytic complex with 2-oxoglutarate, Eur. J. Biochem., 267, 3583–3591.
Bunik, V. I., and Pavlova, O. G. (1993) Inactivation of alpha-ketoglutarate dehydrogenase during oxidative decarboxylation of alpha-ketoadipic acid, FEBS Lett., 323, 166–170.
Goncalves, R. L., Bunik, V. I., and Brand, M. D. (2016) Production of superoxide/hydrogen peroxide by the mitochondrial 2-oxoadipate dehydrogenase complex, Free Radic. Biol. Med., 91, 247–255.
Xu, W., Zhu, H., Gu, M., Luo, Q., Ding, J., Yao, Y., Chen, F., and Wang, Z. (2013) DHTKD1 is essential for mitochondrial biogenesis and function maintenance, FEBS Lett., 587, 3587–3592.
Jia, X., Miao, Z., Li, W., Zhang, L., Feng, C., He, Y., Bi, X., Wang, L., Du, Y., Hou, M., Hao, D., Xiao, Y., Chen, L., and Li, K. (2014) Cancer-risk module identification and module-based disease risk evaluation: a case study on lung cancer, PLoS One, 9, e92395.
Wu, C., Orozco, C., Boyer, J., Leglise, M., Goodale, J., Batalov, S., Hodge, C. L., Haase, J., Janes, J., Huss, J. W., 3rd, and Su, A. I. (2009) BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources, Genome Biol., 10, R130.
GTEx Consortium (2013) The Genotype-Tissue Expression (GTEx) project, Nat. Genet., 45, 580–585.
Xu, W. Y., Gu, M. M., Sun, L. H., Guo, W. T., Zhu, H. B., Ma, J. F., Yuan, W. T., Kuang, Y., Ji, B. J., Wu, X. L., Chen, Y., Zhang, H. X., Sun, F. T., Huang, W., Huang, L., Chen, S. D., and Wang, Z. G. (2012) A nonsense mutation in DHTKD1 causes Charcot–Marie–Tooth disease type 2 in a large Chinese pedigree, Am. J. Hum. Genet., 91, 1088–1094.
Kumaran, S., Patel, M. S., and Jordan, F. (2013) Nuclear magnetic resonance approaches in the study of 2-oxo acid dehydrogenase multienzyme complexes–a literature review, Molecules, 18, 11873–11903.
Danson, M. J., Hooper, E. A., and Perham, R. N. (1978) Intramolecular coupling of active sites in the pyruvate dehydrogenase multienzyme complex of Escherichia coli, Biochem. J., 175, 193–198.
Cate, R. L., Roche, T. E., and Davis, L. C. (1980) Rapid intersite transfer of acetyl groups and movement of pyruvate dehydrogenase component in the kidney pyruvate dehydrogenase complex, J. Biol. Chem., 255, 7556–7562.
Perham, R. N. (1991) Domains, motifs, and linkers in 2oxo acid dehydrogenase multienzyme complexes: a paradigm in the design of a multifunctional protein, Biochemistry, 30, 8501–8512.
Patel, M. S., and Korotchkina, L. G. (2001) Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation: complexity of multiple phosphorylation sites and kinases, Exp. Mol. Med., 33, 191–197.
Mattevi, A., Schierbeek, A. J., and Hol, W. G. (1991) Refined crystal structure of lipoamide dehydrogenase from Azotobacter vinelandii at 2.2 Å resolution. A comparison with the structure of glutathione reductase, J. Mol. Biol., 220, 975–994.
Mattevi, A., Obmolova, G., Kalk, K. H., Van Berkel, W. J., and Hol, W. G. (1993) Three-dimensional structure of lipoamide dehydrogenase from Pseudomonas fluorescens at 2.8 Å resolution. Analysis of redox and thermostability properties, J. Mol. Biol., 230, 1200–1215.
Brautigam, C. A., Chuang, J. L., Tomchick, D. R., Machius, M., and Chuang, D. T. (2005) Crystal structure of human dihydrolipoamide dehydrogenase: NAD+/NADH binding and the structural basis of disease-causing mutations, J. Mol. Biol., 350, 543–552.
Aevarsson, A., Chuang, J. L., Wynn, R. M., Turley, S., Chuang, D. T., and Hol, W. G. (2000) Crystal structure of human branched-chain alpha-ketoacid dehydrogenase and the molecular basis of multienzyme complex deficiency in maple syrup urine disease, Structure, 8, 277–291.
Arjunan, P., Nemeria, N., Brunskill, A., Chandrasekhar, K., Sax, M., Yan, Y., Jordan, F., Guest, J. R., and Furey, W. (2002) Structure of the pyruvate dehydrogenase multienzyme complex E1 component from Escherichia coli at 1.85 Å resolution, Biochemistry, 41, 5213–5221.
Ciszak, E. M., Korotchkina, L. G., Dominiak, P. M., Sidhu, S., and Patel, M. S. (2003) Structural basis for flipflop action of thiamin pyrophosphate-dependent enzymes revealed by human pyruvate dehydrogenase, J. Biol. Chem., 278, 21240–21246.
Frank, R. A., Price, A. J., Northrop, F. D., Perham, R. N., and Luisi, B. F. (2007) Crystal structure of the E1 component of the Escherichia coli 2-oxoglutarate dehydrogenase multienzyme complex, J. Mol. Biol., 368, 639–651.
Kato, M., Wynn, R. M., Chuang, J. L., Tso, S. C., Machius, M., Li, J., and Chuang, D. T. (2008) Structural basis for inactivation of the human pyruvate dehydrogenase complex by phosphorylation: role of disordered phosphorylation loops, Structure, 16, 1849–1859.
Milne, J. L., Shi, D., Rosenthal, P. B., Sunshine, J. S., Domingo, G. J., Wu, X., Brooks, B. R., Perham, R. N., Henderson, R., and Subramaniam, S. (2002) Molecular architecture and mechanism of an icosahedral pyruvate dehydrogenase complex: a multifunctional catalytic machine, EMBO J., 21, 5587–5598.
Gu, Y., Zhou, Z. H., McCarthy, D. B., Reed, L. J., and Stoops, J. K. (2003) 3D electron microscopy reveals the variable deposition and protein dynamics of the peripheral pyruvate dehydrogenase component about the core, Proc. Natl. Acad. Sci. USA, 100, 7015–7020.
Murphy, G. E., and Jensen, G. J. (2005) Electron cryotomography of the E. coli pyruvate and 2-oxoglutarate dehydrogenase complexes, Structure, 13, 1765–1773.
Robien, M. A., Clore, G. M., Omichinski, J. G., Perham, R. N., Appella, E., Sakaguchi, K., and Gronenborn, A. M. (1992) Three-dimensional solution structure of the E3binding domain of the dihydrolipoamide succinyltransferase core from the 2-oxoglutarate dehydrogenase multienzyme complex of Escherichia coli, Biochemistry, 31, 3463–3471.
Mattevi, A., Obmolova, G., Kalk, K. H., Teplyakov, A., and Hol, W. G. (1993) Crystallographic analysis of substrate binding and catalysis in dihydrolipoyl transacetylase (E2p), Biochemistry, 32, 3887–3901.
Mattevi, A., Obmolova, G., Kalk, K. H., Westphal, A. H., De Kok, A., and Hol, W. G. (1993) Refined crystal structure of the catalytic domain of dihydrolipoyl transacetylase (E2p) from Azotobacter vinelandii at 2.6 Å resolution, J. Mol. Biol., 230, 1183–1199.
Kalia, Y. N., Brocklehurst, S. M., Hipps, D. S., Appella, E., Sakaguchi, K., and Perham, R. N. (1993) The high-resolution structure of the peripheral subunit-binding domain of dihydrolipoamide acetyltransferase from the pyruvate dehydrogenase multienzyme complex of Bacillus stearothermophilus, J. Mol. Biol., 230, 323–341.
Dardel, F., Davis, A. L., Laue, E. D., and Perham, R. N. (1993) Three-dimensional structure of the lipoyl domain from Bacillus stearothermophilus pyruvate dehydrogenase multienzyme complex, J. Mol. Biol., 229, 1037–1048.
Green, J. D., Laue, E. D., Perham, R. N., Ali, S. T., and Guest, J. R. (1995) Three-dimensional structure of a lipoyl domain from the dihydrolipoyl acetyltransferase component of the pyruvate dehydrogenase multienzyme complex of Escherichia coli, J. Mol. Biol., 248, 328–343.
Ricaud, P. M., Howard, M. J., Roberts, E. L., Broadhurst, R. W., and Perham, R. N. (1996) Three-dimensional structure of the lipoyl domain from the dihydrolipoyl succinyltransferase component of the 2-oxoglutarate dehydrogenase multienzyme complex of Escherichia coli, J. Mol. Biol., 264, 179–190.
Chang, C. F., Chou, H. T., Chuang, J. L., Chuang, D. T., and Huang, T. H. (2002) Solution structure and dynamics of the lipoic acid-bearing domain of human mitochondrial branched-chain alpha-keto acid dehydrogenase complex, J. Biol. Chem., 277, 15865–15873.
Chang, C. F., Chou, H. T., Lin, Y. J., Lee, S. J., Chuang, J. L., Chuang, D. T., and Huang, T. H. (2006) Structure of the subunit binding domain and dynamics of the di-domain region from the core of human branched chain alphaketoacid dehydrogenase complex, J. Biol. Chem., 281, 28345–28353.
Wang, J., Nemeria, N. S., Chandrasekhar, K., Kumaran, S., Arjunan, P., Reynolds, S., Calero, G., Brukh, R., Kakalis, L., Furey, W., and Jordan, F. (2014) Structure and function of the catalytic domain of the dihydrolipoyl acetyltransferase component in Escherichia coli pyruvate dehydrogenase complex, J. Biol. Chem., 289, 15215–15230.
Jilka, J. M., Rahmatullah, M., Kazemi, M., and Roche, T. E. (1986) Properties of a newly characterized protein of the bovine kidney pyruvate dehydrogenase complex, J. Biol. Chem., 261, 1858–1867.
Smolle, M., Prior, A. E., Brown, A. E., Cooper, A., Byron, O., and Lindsay, J. G. (2006) A new level of architectural complexity in the human pyruvate dehydrogenase complex, J. Biol. Chem., 281, 19772–19780.
Harris, R. A., Bowker-Kinley, M. M., Wu, P., Jeng, J., and Popov, K. M. (1997) Dihydrolipoamide dehydrogenasebinding protein of the human pyruvate dehydrogenase complex. DNA-derived amino acid sequence, expression, and reconstitution of the pyruvate dehydrogenase complex, J. Biol. Chem., 272, 19746–19751.
Behal, R. H., Browning, K. S., Hall, T. B., and Reed, L. J. (1989) Cloning and nucleotide sequence of the gene for protein X from Saccharomyces cerevisiae, Proc. Natl. Acad. Sci. USA, 86, 8732–8736.
Klingbeil, M. M., Walker, D. J., Arnette, R., Sidawy, E., Hayton, K., Komuniecki, P. R., and Komuniecki, R. (1996) Identification of a novel dihydrolipoyl dehydrogenase-binding protein in the pyruvate dehydrogenase complex of the anaerobic parasitic nematode, Ascaris suum, J. Biol. Chem., 271, 5451–5457.
Taylor, A. E., Cogdell, R. J., and Lindsay, J. G. (1992) Immunological comparison of the pyruvate dehydrogenase complexes from pea mitochondria and chloroplasts, Planta, 188, 225–231.
Broz, A. K., Tovar-Mendez, A., Mooney, B. P., Johnston, M. L., Miernyk, J. A., and Randall, D. D. (2014) A novel regulatory mechanism based upon a dynamic core structure for the mitochondrial pyruvate dehydrogenase complex? Mitochondrion, 19, Pt. B, 144–153.
Rice, J. E., Dunbar, B., and Lindsay, J. G. (1992) Sequences directing dihydrolipoamide dehydrogenase (E3) binding are located on the 2-oxoglutarate dehydrogenase (E1) component of the mammalian 2-oxoglutarate dehydrogenase multienzyme complex, EMBO J., 11, 3229–3235.
McCartney, R. G., Rice, J. E., Sanderson, S. J., Bunik, V., Lindsay, H., and Lindsay, J. G. (1998) Subunit interactions in the mammalian alpha-ketoglutarate dehydrogenase complex. Evidence for direct association of the alphaketoglutarate dehydrogenase and dihydrolipoamide dehydrogenase components, J. Biol. Chem., 273, 24158–24164.
Hanemaaijer, R., Westphal, A. H., Van Der Heiden, T., De Kok, A., and Veeger, C. (1989) The quaternary structure of the dihydrolipoyl transacetylase component of the pyruvate dehydrogenase complex from Azotobacter vinelandii. A reconsideration, Eur. J. Biochem., 179, 287–292.
Izard, T., Sarfaty, S., Westphal, A., De Kok, A., and Hol, W. G. (1997) Improvement of diffraction quality upon rehydration of dehydrated icosahedral Enterococcus faecalis pyruvate dehydrogenase core crystals, Protein Sci., 6, 913–915.
De Kok, A., Hengeveld, A. F., Martin, A., and Westphal, A. H. (1998) The pyruvate dehydrogenase multi-enzyme complex from Gram-negative bacteria, Biochim. Biophys. Acta, 1385, 353–366.
Randall, D. D., Miernyk, J. A., Fang, T. K., Budde, R. J., and Schuller, K. A. (1989) Regulation of the pyruvate dehydrogenase complexes in plants, Ann. N. Y. Acad. Sci., 573, 192–205.
Tovar-Mendez, A., Miernyk, J. A., and Randall, D. D. (2003) Regulation of pyruvate dehydrogenase complex activity in plant cells, Eur. J. Biochem., 270, 1043–1049.
Grande, H. J., Bresters, T. W., De Abreu, R. A., De Kok, A., and Veeger, C. (1975) The pyruvate-dehydrogenase complex from Azotobacter vinelandii. 3. Stoichiometry and function of the individual components, Eur. J. Biochem., 59, 355–363.
Bosma, H. J., De Kok, A., Westphal, A. H., and Veeger, C. (1984) The composition of the pyruvate dehydrogenase complex from Azotobacter vinelandii. Does a unifying model exist for the complexes from gram-negative bacteria? Eur. J. Biochem., 142, 541–549.
Schulze, E., Westphal, A. H., Boumans, H., and De Kok, A. (1991) Site-directed mutagenesis of the dihydrolipoyl transacetylase component (E2p) of the pyruvate dehydrogenase complex from Azotobacter vinelandii. Binding of the peripheral components E1p and E3, Eur. J. Biochem., 202, 841–848.
Lessard, I. A., and Perham, R. N. (1995) Interaction of component enzymes with the peripheral subunit-binding domain of the pyruvate dehydrogenase multienzyme complex of Bacillus stearothermophilus: stoichiometry and specificity in self-assembly, Biochem. J., 306 (Pt. 3), 727–733.
Domingo, G. J., Chauhan, H. J., Lessard, I. A., Fuller, C., and Perham, R. N. (1999) Self-assembly and catalytic activity of the pyruvate dehydrogenase multienzyme complex from Bacillus stearothermophilus, Eur. J. Biochem., 266, 1136–1146.
Reed, L. J., Pettit, F. H., Eley, M. H., Hamilton, L., Collins, J. H., and Oliver, R. M. (1975) Reconstitution of the Escherichia coli pyruvate dehydrogenase complex, Proc. Natl. Acad. Sci. USA, 72, 3068–3072.
Jung, H. I., Bowden, S. J., Cooper, A., and Perham, R. N. (2002) Thermodynamic analysis of the binding of component enzymes in the assembly of the pyruvate dehydrogenase multienzyme complex of Bacillus stearothermophilus, Protein Sci., 11, 1091–1100.
Jung, H. I., Cooper, A., and Perham, R. N. (2003) Interactions of the peripheral subunit-binding domain of the dihydrolipoyl acetyltransferase component in the assembly of the pyruvate dehydrogenase multienzyme complex of Bacillus stearothermophilus, Eur. J. Biochem., 270, 4488–4496.
Strumilo, S. (2005) Short-term regulation of the alphaketoglutarate dehydrogenase complex by energy-linked and some other effectors, Biochemistry (Moscow), 70, 726–729.
Strumilo, S. (2005) Short-term regulation of the mammalian pyruvate dehydrogenase complex, Acta Biochim. Pol., 52, 759–764.
Tylicki, A., Bunik, V. I., and Strumilo, S. (2011) 2-Oxoglutarate dehydrogenase complex and its multipoint control, Postepy Biochem., 57, 304–313.
Harris, R. A., Bowker-Kinley, M. M., Huang, B., and Wu, P. (2002) Regulation of the activity of the pyruvate dehydrogenase complex, Adv. Enzyme Regul., 42, 249–259.
Saunier, E., Benelli, C., and Bortoli, S. (2016) The pyruvate dehydrogenase complex in cancer: An old metabolic gatekeeper regulated by new pathways and pharmacological agents, Int. J. Cancer, 138, 809–817.
Obayashi, M., Sato, Y., Harris, R. A., and Shimomura, Y. (2001) Regulation of the activity of branched-chain 2-oxo acid dehydrogenase (BCODH) complex by binding BCODH kinase, FEBS Lett., 491, 50–54.
Armstrong, C. T., Anderson, J. L., and Denton, R. M. (2014) Studies on the regulation of the human E1 subunit of the 2-oxoglutarate dehydrogenase complex, including the identification of a novel calcium-binding site, Biochem. J., 459, 369–381.
Vassylyev, D. G., and Symersky, J. (2007) Crystal structure of pyruvate dehydrogenase phosphatase 1 and its functional implications, J. Mol. Biol., 370, 417–426.
Turkan, A., Hiromasa, Y., and Roche, T. E. (2004) Formation of a complex of the catalytic subunit of pyruvate dehydrogenase phosphatase isoform 1 (PDP1c) and the L2 domain forms a Ca2+ binding site and captures PDP1c as a monomer, Biochemistry, 43, 15073–15085.
Olson, M. S. (1989) Regulation of the mitochondrial multienzyme complexes in complex metabolic systems, Ann. N. Y. Acad. Sci., 573, 218–229.
Bunik, V. I., Raddatz, G., Wanders, R. J., and Reiser, G. (2006) Brain pyruvate and 2-oxoglutarate dehydrogenase complexes are mitochondrial targets of the CoA ester of the Refsum disease marker phytanic acid, FEBS Lett., 580, 3551–3557.
Strumilo, S. A., and Vinogradov, V. V. (1988) Vitamin-Dependent Enzymes of Adrenal Gland (2-Oxo Acid Dehydrogenases) [in Russian], Institute for Biochemistry of the Academy of Sciences of BSSR, Minsk.
Patel, M. S. (1974) Inhibition by the branched-chain 2-oxo acids of the 2-oxoglutarate dehydrogenase complex in developing rat and human brain, Biochem. J., 144, 91–97.
Gudi, R., Bowker-Kinley, M. M., Kedishvili, N. Y., Zhao, Y., and Popov, K. M. (1995) Diversity of the pyruvate dehydrogenase kinase gene family in humans, J. Biol. Chem., 270, 28989–28994.
Huang, B., Gudi, R., Wu, P., Harris, R. A., Hamilton, J., and Popov, K. M. (1998) Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation, J. Biol. Chem., 273, 17680–17688.
Roche, T. E., Baker, J. C., Yan, X., Hiromasa, Y., Gong, X., Peng, T., Dong, J., Turkan, A., and Kasten, S. A. (2001) Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms, Prog. Nucleic Acid Res. Mol. Biol., 70, 33–75.
Damuni, Z., Merryfield, M. L., Humphreys, J. S., and Reed, L. J. (1984) Purification and properties of branchedchain alpha-keto acid dehydrogenase phosphatase from bovine kidney, Proc. Natl. Acad. Sci. USA, 81, 4335–4338.
Huang, Y., and Chuang, D. T. (1996) Structural organization of the rat branched-chain 2-oxo-acid dehydrogenase kinase gene and partial characterization of the promoterregulatory region, Biochem. J., 313 (Pt. 2), 603–609.
Harris, R. A., Joshi, M., Jeoung, N. H., and Obayashi, M. (2005) Overview of the molecular and biochemical basis of branched-chain amino acid catabolism, J. Nutr., 135, 1527S–1530S.
Shimomura, Y., Honda, T., Shiraki, M., Murakami, T., Sato, J., Kobayashi, H., Mawatari, K., Obayashi, M., and Harris, R. A. (2006) Branched-chain amino acid catabolism in exercise and liver disease, J. Nutr., 136, 250S–253S.
Zhou, M., Lu, G., Gao, C., Wang, Y., and Sun, H. (2012) Tissue-specific and nutrient regulation of the branchedchain alpha-keto acid dehydrogenase phosphatase, protein phosphatase 2Cm (PP2Cm), J. Biol. Chem., 287, 23397–23406.
Oyarzabal, A., Martinez-Pardo, M., Merinero, B., Navarrete, R., Desviat, L. R., Ugarte, M., and Rodriguez-Pombo, P. (2013) A novel regulatory defect in the branched-chain alpha-keto acid dehydrogenase complex due to a mutation in the PPM1K gene causes a mild variant phenotype of maple syrup urine disease, Hum. Mutat., 34, 355–362.
Oyarzabal, A., Bravo-Alonso, I., Sanchez-Arago, M., Rejas, M. T., Merinero, B., Garcia-Cazorla, A., Artuch, R., Ugarte, M., and Rodriguez-Pombo, P. (2016) Mitochondrial response to the BCKDK-deficiency: some clues to understand the positive dietary response in this form of autism, Biochim. Biophys. Acta, 1862, 592–600.
Yan, J., Lawson, J. E., and Reed, L. J. (1996) Role of the regulatory subunit of bovine pyruvate dehydrogenase phosphatase, Proc. Natl. Acad. Sci. USA, 93, 4953–4956.
Lawson, J. E., Park, S. H., Mattison, A. R., Yan, J., and Reed, L. J. (1997) Cloning, expression, and properties of the regulatory subunit of bovine pyruvate dehydrogenase phosphatase, J. Biol. Chem., 272, 31625–31629.
Damuni, Z., Humphreys, J. S., and Reed, L. J. (1984) Stimulation of pyruvate dehydrogenase phosphatase activity by polyamines, Biochem. Biophys. Res. Commun., 124, 95–99.
Damuni, Z., Lim Tung, H. Y., and Reed, L. J. (1985) Specificity of the heat-stable protein inhibitor of the branched-chain alpha-keto acid dehydrogenase phosphatase, Biochem. Biophys. Res. Commun., 133, 878–883.
Damuni, Z., Humphreys, J. S., and Reed, L. J. (1986) A potent, heat-stable protein inhibitor of [branched-chain alpha-keto acid dehydrogenase]-phosphatase from bovine kidney mitochondria, Proc. Natl. Acad. Sci. USA, 83, 285–289.
Damuni, Z., and Reed, L. J. (1988) Branched-chain alpha-keto acid dehydrogenase phosphatase and its inhibitor protein from bovine kidney, Methods Enzymol., 166, 321–329.
Fan, J., Kang, H. B., Shan, C., Elf, S., Lin, R., Xie, J., Gu, T. L., Aguiar, M., Lonning, S., Chung, T. W., Arellano, M., Khoury, H. J., Shin, D. M., Khuri, F. R., Boggon, T. J., Kang, S., and Chen, J. (2014) Tyr-301 phosphorylation inhibits pyruvate dehydrogenase by blocking substrate binding and promotes the Warburg effect, J. Biol. Chem., 289, 26533–26541.
Hitosugi, T., Fan, J., Chung, T. W., Lythgoe, K., Wang, X., Xie, J., Ge, Q., Gu, T. L., Polakiewicz, R. D., Roesel, J. L., Chen, G. Z., Boggon, T. J., Lonial, S., Fu, H., Khuri, F. R., Kang, S., and Chen, J. (2011) Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism, Mol. Cell, 44, 864–877.
Fan, J., Shan, C., Kang, H. B., Elf, S., Xie, J., Tucker, M., Gu, T. L., Aguiar, M., Lonning, S., Chen, H., Mohammadi, M., Britton, L. M., Garcia, B. A., Aleckovic, M., Kang, Y., Kaluz, S., Devi, N., Van Meir, E. G., Hitosugi, T., Seo, J. H., Lonial, S., Gaddh, M., Arellano, M., Khoury, H. J., Khuri, F. R., Boggon, T. J., Kang, S., and Chen, J. (2014) Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex, Mol. Cell, 53, 534–548.
Shan, C., Kang, H. B., Elf, S., Xie, J., Gu, T. L., Aguiar, M., Lonning, S., Hitosugi, T., Chung, T. W., Arellano, M., Khoury, H. J., Shin, D. M., Khuri, F. R., Boggon, T. J., and Fan, J. (2014) Tyr-94 phosphorylation inhibits pyruvate dehydrogenase phosphatase 1 and promotes tumor growth, J. Biol. Chem., 289, 21413–21422.
Cairns, R. A., Harris, I. S., and Mak, T. W. (2011) Regulation of cancer cell metabolism, Nat. Rev. Cancer, 11, 85–95.
Jha, M. K., and Suk, K. (2013) Pyruvate dehydrogenase kinase as a potential therapeutic target for malignant gliomas, Brain Tumor Res. Treat., 1, 57–63.
Bingham, P. M., Stuart, S. D., and Zachar, Z. (2014) Lipoic acid and lipoic acid analogs in cancer metabolism and chemotherapy, Expert Rev. Clin. Pharmacol., 7, 837–846.
Ozden, O., Park, S. H., Wagner, B. A., Yong Song, H., Zhu, Y., Vassilopoulos, A., Jung, B., Buettner, G. R., and Gius, D. (2014) SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells, Free Radic. Biol. Med., 76, 163–172.
Park, J., Chen, Y., Tishkoff, D. X., Peng, C., Tan, M., Dai, L., Xie, Z., Zhang, Y., Zwaans, B. M., Skinner, M. E., Lombard, D. B., and Zhao, Y. (2013) SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways, Mol. Cell, 50, 919–930.
Gibson, G. E., Xu, H., Chen, H. L., Chen, W., Denton, T. T., and Zhang, S. (2015) Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines, J. Neurochem., 134, 86–96.
Barry, G. F. (2000) Phosphonate metabolizing plants, in Google Patents, US Patent No. WO/2000/029596.
Zhang, G., Allen, K. N., and Dunaway-Mariano, D. (2003) Enzymatic synthesis of radiolabeled phosphonoacetaldehyde, Anal. Biochem., 322, 233–237.
Olsen, D. B., Hepburn, T. W., Moos, M., Mariano, P. S., and Dunaway-Mariano, D. (1988) Investigation of the Bacillus cereus phosphonoacetaldehyde hydrolase. Evidence for a Schiff base mechanism and sequence analysis of an active-site peptide containing the catalytic lysine residue, Biochemistry, 27, 2229–2234.
Zhang, G., Dai, J., Lu, Z., and Dunaway-Mariano, D. (2003) The phosphonopyruvate decarboxylase from Bacteroides fragilis, J. Biol. Chem., 278, 41302–41308.
Nakashita, H., Kozuka, K., Hidaka, T., Hara, O., and Seto, H. (2000) Identification and expression of the gene encoding phosphonopyruvate decarboxylase of Streptomyces hygroscopicus, Biochim. Biophys. Acta, 1490, 159–162.
Chang, W. C., Dey, M., Liu, P., Mansoorabadi, S. O., Moon, S. J., Zhao, Z. K., Drennan, C. L., and Liu, H. W. (2013) Mechanistic studies of an unprecedented enzymecatalyzed 1,2-phosphono-migration reaction, Nature, 496, 114–118.
Kamat, S. S., Williams, H. J., and Raushel, F. M. (2011) Intermediates in the transformation of phosphonates to phosphate by bacteria, Nature, 480, 570–573.
Kononova, S. V., and Nesmeyanova, M. A. (2002) Phosphonates and their degradation by microorganisms, Biochemistry (Moscow), 67, 184–195.
Laber, B., and Amrhein, N. (1987) Metabolism of 1aminoethylphosphinate generates acetylphosphinate, a potent inhibitor of pyruvate dehydrogenase, Biochem. J., 248, 351–358.
Peck, S. C., Gao, J., and Van der Donk, W. A. (2012) Discovery and biosynthesis of phosphonate and phosphinate natural products, Methods Enzymol., 516, 101–123.
He, H. W., Peng, H., and Tan, X. S. (2014) Environmentally Friendly Alkylphosphonate Herbicides, Chemical Industry Press, Beijing.
Eto, M. (1997) Functions of phosphorus moiety in agrochemical molecules, Biosci. Biotech. Biochem., 61, 1–11.
Engel, R. (1977) Phosphonates as analogues of natural phosphates, Chem. Rev., 77, 349–367.
Vvedensky, V. (1888) Some data on structure of phosphoric acid, Zh. Russ. Fiz. Khim. Obshch., 20, 29–34.
Kabachnik, M. I., and Rossiyskaya, P. A. (1945) Esters of α-ketophosphinic acids, Izv. Akad. Nauk SSSR Ser. Khim., 4, 364–374.
Kreutzkamp, N., and Mengel, W. (1967) Darstellung einiger γ- und α-dicarbonyl-phosphonsaure-ester. 10. Mitt. uber carbonyl- und cyan-phosphonester, Arch. Pharm., 300, 389–392.
Kluger, R., and Pike, D. C. (1977) Active site generated analogues of reactive intermediates in enzymic reactions. Potent inhibition of pyruvate dehydrogenase by a phosphonate analogue of pyruvate, J. Am. Chem. Soc., 99, 4504–4506.
Khomutov, R. M., Osipova, T. I., and Zhukov, Y. N. (1978) Synthesis of alpha-ketophosphonic acids, Bull. Acad. Sci. USSR. Div. Chem. Sci., 27, 1210–1213.
Baillie, A. C., Wright, K., Wright, B. J., and Earnshaw, C. G. (1988) Inhibitors of pyruvate dehydrogenase as herbicides, Pestic. Biochem. Physiol., 30, 103–112.
Kluger, R., and Pike, D. C. (1979) Chemical synthesis of a proposed enzyme-generated “reactive intermediate analogue” derived from thiamin diphosphate. Self-activation of pyruvate dehydrogenase by conversion of the analogue to its components, J. Am. Chem. Soc., 101, 6425–6428.
Biryukov, A. I., Bunik, V. I., Zhukov, Y. N., Khurs, E. N., and Khomutov, R. M. (1996) Succinyl phosphonate inhibits alpha-ketoglutarate oxidative decarboxylation, catalyzed by alpha-ketoglutarate dehydrogenase complexes from E. coli and pigeon breast muscle, FEBS Lett., 382, 167–170.
Fang, M., Toogood, R. D., Macova, A., Ho, K., Franzblau, S. G., McNeil, M. R., Sanders, D. A., and Palmer, D. R. (2010) Succinylphosphonate esters are competitive inhibitors of MenD that show active-site discrimination between homologous alpha-ketoglutarate-decarboxylating enzymes, Biochemistry, 49, 2672–2679.
Bunik, V. I., and Fernie, A. R. (2009) Metabolic control exerted by the 2-oxoglutarate dehydrogenase reaction: a cross-kingdom comparison of the crossroad between energy production and nitrogen assimilation, Biochem. J., 422, 405–421.
Nemeria, N. S., Korotchkina, L. G., Chakraborty, S., Patel, M. S., and Jordan, F. (2006) Acetylphosphinate is the most potent mechanism-based substrate-like inhibitor of both the human and Escherichia coli pyruvate dehydrogenase components of the pyruvate dehydrogenase complex, Bioorg. Chem., 34, 362–379.
Arjunan, P., Sax, M., Brunskill, A., Chandrasekhar, K., Nemeria, N., Zhang, S., Jordan, F., and Furey, W. (2006) A thiamin-bound, pre-decarboxylation reaction intermediate analogue in the pyruvate dehydrogenase E1 subunit induces large scale disorder-to-order transformations in the enzyme and reveals novel structural features in the covalently bound adduct, J. Biol. Chem., 281, 15296–15303.
Karaman, R., Goldblum, A., Breuer, E., and Leader, H. J. (1989) Acylphosphonic acids and methyl hydrogen acylphosphonates: physical and chemical properties and theoretical calculations, J. Chem. Soc. Perkin Trans., 1, 765–774.
Bunik, V. I., Biryukov, A. I., and Zhukov, Yu. N. (1992) Inhibition of pigeon breast muscle alpha-ketoglutarate dehydrogenase by phosphonate analogues of alpha-ketoglutarate, FEBS Lett., 303, 197–201.
Bunik, V. I., and Pavlova, O. G. (1997) Inhibition of pigeon breast muscle alpha-ketoglutarate dehydrogenase by structural analogs of alpha-ketoglutarate, Biochemistry (Moscow), 62, 1012–1020.
Nemeria, N., Baykal, A., Joseph, E., Zhang, S., Yan, Y., Furey, W., and Jordan, F. (2004) Tetrahedral intermediates in thiamin diphosphate-dependent decarboxylations exist as a 1′,4′-imino tautomeric form of the coenzyme, unlike the Michaelis complex or the free coenzyme, Biochemistry, 43, 6565–6575.
Kale, S., Ulas, G., Song, J., Brudvig, G. W., Furey, W., and Jordan, F. (2008) Efficient coupling of catalysis and dynamics in the E1 component of Escherichia coli pyruvate dehydrogenase multienzyme complex, Proc. Natl. Acad. Sci. USA, 105, 1158–1163.
Kale, S., and Jordan, F. (2009) Conformational ensemble modulates cooperativity in the rate-determining catalytic step in the E1 component of the Escherichia coli pyruvate dehydrogenase multienzyme complex, J. Biol. Chem., 284, 33122–33129.
Nemeria, N. S., Arjunan, P., Chandrasekhar, K., Mossad, M., Tittmann, K., Furey, W., and Jordan, F. (2010) Communication between thiamin cofactors in the Escherichia coli pyruvate dehydrogenase complex E1 component active centers: evidence for a “direct pathway” between the 4′-aminopyrimidine N1′ atoms, J. Biol. Chem., 285, 11197–11209.
Fang, M., Macova, A., Hanson, K. L., Kos, J., and Palmer, D. R. (2011) Using substrate analogues to probe the kinetic mechanism and active site of Escherichia coli MenD, Biochemistry, 50, 8712–8721.
Bunik, V. I., Denton, T. T., Xu, H., Thompson, C. M., Cooper, A. J., and Gibson, G. E. (2005) Phosphonate analogues of alpha-ketoglutarate inhibit the activity of the alpha-ketoglutarate dehydrogenase complex isolated from brain and in cultured cells, Biochemistry, 44, 10552–10561.
Bera, A. K., Polovnikova, L. S., Roestamadji, J., Widlanski, T. S., Kenyon, G. L., McLeish, M. J., and Hasson, M. S. (2007) Mechanism-based inactivation of benzoylformate decarboxylase, a thiamin diphosphatedependent enzyme, J. Am. Chem. Soc., 129, 4120–4121.
Schonbrunn-Hanebeck, E., Laber, B., and Amrhein, N. (1990) Slow-binding inhibition of the Escherichia coli pyruvate dehydrogenase multienzyme complex by acetylphosphinate, Biochemistry, 29, 4880–4885.
Bunik, V. I., Artiukhov, A., Kazantsev, A., Goncalves, R., Daloso, D., Oppermann, H., Kulakovskaya, E., Lukashev, N., Fernie, A., Brand, M., and Gaunitz, F. (2015) Specific inhibition by synthetic analogs of pyruvate reveals that the pyruvate dehydrogenase reaction is essential for metabolism and viability of glioblastoma cells, Oncotarget, 6, 40036–40052.
Da Shim, J., Nemeria, N. S., Balakrishnan, A., Patel, H., Song, J., Wang, J., Jordan, F., and Farinas, E. T. (2011) Assignment of function to histidines 260 and 298 by engineering the E1 component of the Escherichia coli 2-oxoglutarate dehydrogenase complex; substitutions that lead to acceptance of substrates lacking the 5-carboxyl group, Biochemistry, 50, 7705–7709.
Araujo, W. L., Nunes-Nesi, A., Trenkamp, S., Bunik, V. I., and Fernie, A. R. (2008) Inhibition of 2-oxoglutarate dehydrogenase in potato tuber suggests the enzyme is limiting for respiration and confirms its importance in nitrogen assimilation, Plant Physiol., 148, 1782–1796.
O’Brien, T. A., Kluger, R., Pike, D. C., and Gennis, R. B. (1980) Phosphonate analogues of pyruvate. Probes of substrate binding to pyruvate oxidase and other thiamin pyrophosphate-dependent decarboxylases, Biochim. Biophys. Acta, 613, 10–17.
Dixon, H. B., Giddens, R. A., Harrison, R. A., Henderson, C. E., Norris, W. E., Parker, D. M., Perham, R. N., Slater, P., and Sparkes, M. J. (1991) A synthesis of acylphosphonic acids and of 1-aminoalkylphosphonic acids: the action of pyruvate dehydrogenase and lactate dehydrogenase on acetylphosphonic acid, J. Enzyme Inhib., 5, 111–117.
Araujo, W. L., Tohge, T., Nunes-Nesi, A., Daloso, D. M., Nimick, M., Krahnert, I., Bunik, V. I., Moorhead, G. B., and Fernie, A. R. (2012) Phosphonate analogs of 2-oxoglutarate perturb metabolism and gene expression in illuminated Arabidopsis leaves, Front. Plant Sci., 3, 114.
Van der Merwe, M. J., Osorio, S., Araujo, W. L., Balbo, I., Nunes-Nesi, A., Maximova, E., Carrari, F., Bunik, V. I., Persson, S., and Fernie, A. R. (2010) Tricarboxylic acid cycle activity regulates tomato root growth via effects on secondary cell wall production, Plant Physiol., 153, 611–621.
Bunik, V. I., Kabysheva, M. S., Klimuk, E. I., Storozhevykh, T. P., and Pinelis, V. G. (2009) Phosphono analogues of 2-oxoglutarate protect cerebellar granule neurons upon glutamate excitotoxicity, Ann. N. Y. Acad. Sci., 1171, 521–529.
Araujo, W. L., Trofimova, L., Mkrtchyan, G., Steinhauser, D., Krall, L., Graf, A., Fernie, A. R., and Bunik, V. I. (2013) On the role of the mitochondrial 2-oxoglutarate dehydrogenase complex in amino acid metabolism, Amino Acids, 44, 683–700.
Aleshin, V. A., Artiukhov, A. V., Oppermann, H., Kazantsev, A. V., Lukashev, N. V., and Bunik, V. I. (2015) Mitochondrial impairment may increase cellular NAD(P)H: resazurin oxidoreductase activity, perturbing the NAD(P)H-based viability assays, Cells, 4, 427–451.
Zundorf, G., Kahlert, S., Bunik, V. I., and Reiser, G. (2009) Alpha-ketoglutarate dehydrogenase contributes to production of reactive oxygen species in glutamate-stimulated hippocampal neurons in situ, Neuroscience, 158, 610–616.
Santos, S. S., Gibson, G. E., Cooper, A. J., Denton, T. T., Thompson, C. M., Bunik, V. I., Alves, P. M., and Sonnewald, U. (2006) Inhibitors of the alpha-ketoglutarate dehydrogenase complex alter [1-13C]glucose and [U-13C]glutamate metabolism in cerebellar granule neurons, J. Neurosci. Res., 83, 450–458.
Diaz-Munoz, M. D., Bell, S. E., Fairfax, K., Monzon-Casanova, E., Cunningham, A. F., Gonzalez-Porta, M., Andrews, S. R., Bunik, V. I., Zarnack, K., Curk, T., Heggermont, W. A., Heymans, S., Gibson, G. E., Kontoyiannis, D. L., Ule, J., and Turner, M. (2015) The RNA-binding protein HuR is essential for the B cell antibody response, Nat. Immunol., 16, 415–425.
Kabysheva, M. S., Storozhevykh, T. P., Pinelis, V. G., and Bunik, V. I. (2009) Synthetic regulators of the 2-oxoglutarate oxidative decarboxylation alleviate the glutamate excitotoxicity in cerebellar granule neurons, Biochem. Pharmacol., 77, 1531–1540.
Circello, B. T., Miller, C. G., Lee, J. H., Van der Donk, W. A., and Metcalf, W. W. (2011) The antibiotic dehydrophos is converted to a toxic pyruvate analog by peptide bond cleavage in Salmonella enterica, Antimicrob. Agents Chemother., 55, 3357–3362.
Trofimova, L. K., Araujo, W. L., Strokina, A. A., Fernie, A. R., Bettendorff, L., and Bunik, V. I. (2012) Consequences of the alpha-ketoglutarate dehydrogenase inhibition for neuronal metabolism and survival: implications for neurodegenerative diseases, Curr. Med. Chem., 19, 5895–5906.
Bunik, V., Mkrtchyan, G., Grabarska, A., Oppermann, H., Daloso, D., Araujo, W. L., Juszczak, M., Rzeski, W., Bettendorff, L., Fernie, A. R., Meixensberger, J., Stepulak, A., and Gaunitz, F. (2016) Inhibition of mitochondrial 2-oxoglutarate dehydrogenase impairs viability of cancer cells in a cell-specific metabolism-dependent manner, Oncotarget, 7, 26400–26421.
Graf, A., Trofimova, L., Loshinskaja, A., Mkrtchyan, G., Strokina, A., Lovat, M., Tylicky, A., Strumilo, S., Bettendorff, L., and Bunik, V. I. (2013) Up-regulation of 2-oxoglutarate dehydrogenase as a stress response, Int. J. Biochem. Cell Biol., 45, 175–189.
Araujo, W. L., Tohge, T., Osorio, S., Lohse, M., Balbo, I., Krahnert, I., Sienkiewicz-Porzucek, A., Usadel, B., Nunes-Nesi, A., and Fernie, A. R. (2012) Antisense inhibition of the 2-oxoglutarate dehydrogenase complex in tomato demonstrates its importance for plant respiration and during leaf senescence and fruit maturation, Plant Cell, 24, 2328–2351.
Shi, Q., Risa, O., Sonnewald, U., and Gibson, G. E. (2009) Mild reduction in the activity of the alpha-ketoglutarate dehydrogenase complex elevates GABA shunt and glycolysis, J. Neurochem., 109 (Suppl. 1), 214–221.
Cheshchevik, V., Janssen, A. J. M., Dremza, I. K., Zavodnik, I. B., and Bunik, V. I. (2010) The OGDHCexerted control of mitochondrial respiration is increased under energy demand, in Proc. the MiP 2010 and FEBS Workshop Mitochondrial Physiology (Renner-Sattler, K., and Gnaiger, E., eds.) Obergurgl, Tyrol, Austria, pp. 76–77.
Quinlan, C. L., Goncalves, R. L., Hey-Mogensen, M., Yadava, N., Bunik, V. I., and Brand, M. D. (2014) The 2oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I, J. Biol. Chem., 289, 8312–8325.
Bunik, V. I., and Sievers, C. (2002) Inactivation of the 2oxo acid dehydrogenase complexes upon generation of intrinsic radical species, Eur. J. Biochem., 269, 5004–5015.
Negovsky, V. A., Gurvich, A. M., and Zolotokrylina, E. S. (1987) Post-resuscitation Disease [in Russian], Meditsina, Moscow.
Korge, P., Calmettes, G., and Weiss, J. N. (2016) Reactive oxygen species production in cardiac mitochondria after complex I inhibition: modulation by substrate-dependent regulation of the NADH/NAD(+) ratio, Free Radic. Biol. Med., 96, 22–33.
Mathieu, L., Costa, A. L., Le Bachelier, C., Slama, A., Lebre, A. S., Taylor, R. W., Bastin, J., and Djouadi, F. (2016) Resveratrol attenuates oxidative stress in mitochondrial complex I deficiency: involvement of SIRT3, Free Radic. Biol. Med., 96, 190–198.
Fujikawa, D. G. (2015) The role of excitotoxic programmed necrosis in acute brain injury, Comput. Struct. Biotechnol. J., 13, 212–221.
Pardo, B., Contreras, L., Serrano, A., Ramos, M., Kobayashi, K., Iijima, M., Saheki, T., and Satrustegui, J. (2006) Essential role of aralar in the transduction of small Ca2+ signals to neuronal mitochondria, J. Biol. Chem., 281, 1039–1047.
Bunik, V. I. (2003) 2-Oxo acid dehydrogenase complexes in redox regulation, Eur. J. Biochem., 270, 1036–1042.
Graff, C. L., and Pollack, G. M. (2004) Drug transport at the blood–brain barrier and the choroid plexus, Curr. Drug Metab., 5, 95–108.
Badhan, R. K., Kaur, M., Lungare, S., and Obuobi, S. (2014) Improving brain drug targeting through exploitation of the nose-to-brain route: a physiological and pharmacokinetic perspective, Curr. Drug Deliv., 11, 458–471.
Djupesland, P. G., Messina, J. C., and Mahmoud, R. A. (2014) The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview, Ther. Deliv., 5, 709–733.
Trofimova, L., Lovat, M., Groznaya, A., Efimova, E., Dunaeva, T., Maslova, M., Graf, A., and Bunik, V. (2010) Behavioral impact of the regulation of the brain 2-oxoglutarate dehydrogenase complex by synthetic phosphonate analog of 2-oxoglutarate: implications into the role of the complex in neurodegenerative diseases, Int. J. Alzheimer’s Dis., 749061.
Kulakovskaya, E. A., Loshinskaya, A. A., Graf, A. V., Trofimova, L. K., Maslova, M. V., Bunik, V. I., and Sokolova, N. A. (2012) Effects of acute hypoxia and 2oxoglutarate dehydrogenase complex (OGDHC) inhibitor on cardiac performance in male and female rats, in: IV Congr. of Russian Pharmacologists “Innovations in Modern Pharmacology”, Content of the Congress (Kharkevich, D. A., and Seredenin, S. B., eds.) Kazan, Russia.
Graf, A., Kabysheva, M., Klimuk, E., Trofimova, L., Dunaeva, T., Zundorf, G., Kahlert, S., Reiser, G., Storozhevykh, T., Pinelis, V., Sokolova, N., and Bunik, V. (2009) Role of 2-oxoglutarate dehydrogenase in brain pathologies involving glutamate neurotoxicity, J. Mol. Catal. B Enzym., 61, 80–87.
Mkrtchyan, G., Graf, A., Trofimova, L., and Bunik, V. (2014) Strong positive correlation between the activity of the 2-oxoglutarate dehydrogenase complex and glutamate level in rat cortex disappears after metabolic stress, in Parkinson’s Disease: Genetics, Mechanisms and Therapeutics and the Joint Meeting on Alzheimer’s Disease–From Fundamental Insights to Light at the End of Translational Tunnel (Trojanowsky, J. Q., Albright, C. F., and Zheng, H., eds.) Keystone, Colorado, USA, p. 104.
Bunik, V. I., Schloss, J. V., Pinto, J. T., Dudareva, N., and Cooper, A. J. (2011) A survey of oxidative paracatalytic reactions catalyzed by enzymes that generate carbanionic intermediates: implications for ROS production, cancer etiology, and neurodegenerative diseases, Adv. Enzymol. Relat. Areas Mol. Biol., 77, 307–360.
Gibson, G. E., Blass, J. P., Beal, M. F., and Bunik, V. (2005) The alpha-ketoglutarate–dehydrogenase complex: a mediator between mitochondria and oxidative stress in neurodegeneration, Mol. Neurobiol., 31, 43–63.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2016, Vol. 81, No. 12, pp. 1793–1818.
Rights and permissions
About this article
Cite this article
Artiukhov, A.V., Graf, A.V. & Bunik, V.I. Directed regulation of multienzyme complexes of 2-oxo acid dehydrogenases using phosphonate and phosphinate analogs of 2-oxo acids. Biochemistry Moscow 81, 1498–1521 (2016). https://doi.org/10.1134/S0006297916120129
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297916120129