Abstract
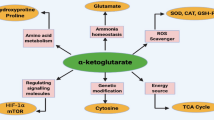
The question of regulation of α-ketoglutarate dehydrogenase complex (KGDHC) has been considered in the biochemical literature very rarely. Moreover, such information is not usually accurate, especially in biochemical textbooks. From the mini-review of research works published during the last 25 years, the following basic view is clear: a) animal KGDHC is very sensitive to ADP, Pi, and Ca2+; b) these positive effectors increase manifold the affinity of KGDHC to α-ketoglutarate; c) KGDHC is inhibited by ATP, NADH, and succinyl-CoA; d) the ATP effect is realized in several ways, probably mainly via opposition versus ADP activation; e) NADH, besides inhibiting dihydrolipoamide dehydrogenase component competitively versus NAD+, decreases the affinity of α-ketoglutarate dehydrogenase to substrate and inactivates it; f) thioredoxin protects KGDHC from self-inactivation during catalysis; g) bacterial and plant KGDHC is activated by AMP instead of ADP. These main effects form the basis of short-term regulation of KGDHC.
Similar content being viewed by others
REFERENCES
Khailova, L. S., and Gomazkova, V. S. (1986) Biokhimiya, 51, 2054–2074.
Campbell, M. K., and Farrell, S. O. (2003) Biochemistry, 4th Edn., Thomson Learning, USA.
Stryer, L. (2002) Biochemistry, 5th Edn., W. H. Freeman, New York.
Nelson, D. L., and Cox, M. M. (2000) Lehninger Principles of Biochemistry, 3rd Edn., Worth Publishers, USA.
Zubay, G. L. (1998) Biochemistry, 4th Edn., Mc Graw Hill, Boston.
Grisham, C. M., and Garrett, R. H. (1998) Biochemistry, 2nd Edn., Brooks Cole, USA.
Matthews, H. R., Freedland, R. A., and Miesfeld, R. L. (1997) Biochemistry: a Short Course, John Wiley & Sons, USA.
Devlin, T. M. (1997) Textbook of Biochemistry, 4th Edn., Wiley Liss, USA.
Hames, B. D., Hooper, N. M., and Houghton, J. D. (1997) Instant Notes in Biochemistry, Bios Scientific Publishers, Oxford, England.
Price, N. C., and Stevens, L. (2001) Fundamentals of Enzymology, 3rd Edn., University Press, Oxford.
Patel, M. S., and Korotchkina, L. G. (2003) BAMBED, 31, 5–15.
Harris, R. A., Bowker-Kinley, M. M., Huang, B., and Wu, P. (2002) Advan. Enzyme Regul., 42, 249–259.
Cooney, G. J., Taegtmeyer, H., and Newsholme, E. A. (1981) Biochem. J., 200, 701–703.
Gibson, G. E., Park, L. C. H., Sheu, K.-F. R., Blass, J. P., and Calingasan, N. Y. (2000) Neurochem. Int., 36, 97–112.
Yeaman, S. J. (1989) Biochem. J., 257, 625–632.
McCormack, J. G., and Denton R. M. (1979) Biochem. J., 180, 533–544.
Gomazkova, V. S., and Krasovskaya, O. E. (1979) Biokhimiya, 44, 1126–1136.
Strumilo, S. A., Vinogradov, V. V., and Senkevich, S. B. (1980) Ukr. Biochem. J., 52, 321–324.
Lawlis, B. V., and Roche, T. E. (1981) Biochemistry, 20, 2512–2524.
McCormack, J. G., and Denton, R. M. (1981) Biochem. J., 196, 619–624.
Ostrovtsova, S. A., and Strumilo, S. A. (1990) Biomed. Biochim. Acta, 49, 515–517.
Nichols, B. J., Rigoulet, M., and Denton, R. M. (1994) Biochem. J., 303, 461–465.
Rodriguez-Zavala, J. S., Pardo, J. P., and Moreno-Sanchez, R. (2000) Arch. Biochem. Biophys., 379, 78–84.
Stafeeva, O. A., Gomazkova, V. S., and Severin, S. E. (1980) Doklady AN USSR, 125, 497–499.
Strumilo, S. A., Taranda, N. I., and Vinogradov, V. V. (1984) Biomed. Biochim. Acta, 43, 237–240.
Strumilo, S. A., Taranda, N. I., and Vinogradov, V. V. (1982) Biokhimiya, 47, 724–732.
Strumilo, S. A., Taranda, N. I., and Vinogradov, V. V. (1983) Doklady AN BSSR, 27, 269–271.
Strumilo, S. A., Taranda, N. I., and Vinogradov, V. V. (1981) Biokhimiya, 46, 156–161.
Markiewicz, J., and Strumilo, S. (1995) Acta Biochim. Polon., 42, 339–346.
Strumilo, S. A. (1988) Voprosy Med. Khim., 34, 2–7.
Weitzman, P. D. J. (1972) FEBS Lett., 22, 323–326.
Wedding, R. T., and Black, M. K. (1971) J. Biol. Chem., 246, 1638–1643.
Bunik, V. I., Buneeva, O. A., and Gomazkova, V. S. (1990) FEBS Lett., 269, 252–254.
Bunik, V., and Sievers, C. (2002) Eur. J. Biochem., 269, 5004–5015.
Smith, C. M., Bryla, J., and Williamson, J. R. (1974) J. Biol. Chem., 249, 1497–1505.
Strumilo, S. A. (1983) Ukr. Biochem. J., 55, 415–419.
Markiewicz, J., and Strumilo, S. (1997) Biochem. Arch., 13, 127–129.
Pawelczyk, T., and Angielski, S. (1984) Acta Biochim. Polon., 31, 289–305.
Bunik, V. I., Buneeva, O. A., and Gomazkova, V. S. (1990) Biochem. Int., 21, 873–881.
Bunik, V., Follmann, H., and Bisswanger, H. (1997) Biol. Chem., 378, 1125–1130.
Bunik, V. I. (2003) Eur. J. Biochem., 270, 1036–1042.
Strumilo, S., and Markiewicz, J. (1995) Biochem. Mol. Biol. Int., 37, 101–106.
Strumilo, S., Czygier, M., Kondracikowska, J., Dobrzyn, P., and Czerniecki, J. (2002) J. Mol. Struct., 614, 221–226.
Author information
Authors and Affiliations
Additional information
__________
Translated from Biokhimiya, Vol. 70, No. 7, 2005, pp. 880–884.
Original Russian Text Copyright © 2005 by Strumilo.
Rights and permissions
About this article
Cite this article
Strumilo, S. Short-Term Regulation of the α-Ketoglutarate Dehydrogenase Complex by Energy-Linked and Some Other Effectors. Biochemistry (Moscow) 70, 726–729 (2005). https://doi.org/10.1007/s10541-005-0177-1
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s10541-005-0177-1