Abstract
Sulphur doping effects on the crystal structures, thermoelectric properties, density-of-states, and effective mass in Cu1.98SxSe1−x were studied based on the electrical and thermal transport property measurements, and first-principles calculations. The X-ray diffraction patterns and Rietveld refinements indicate that room temperature Cu1.98SxSe1−x (x = 0, 0.02, 0.08, 0.16) and Cu1.98SxSe1−x (x = 0.8, 0.9, 1.0) have the same crystal structure as monoclinic-Cu2Se and orthorhombic-Cu2S, respectively. Sulphur doping can greatly enhance zT values when x is in the range of 0.8≤ × ≤1.0. Furthermore, all doped samples show stable thermoelectric compatibility factors over a broad temperature range from 700 to 1000 K, which could greatly benefit their practical applications. First-principles calculations indicate that both the electron density-of-sates and the effective mass for all the compounds exhibit non-monotonic sulphur doping dependence. It is concluded that the overall thermoelectric performance of the Cu1.98SxSe1−x system is mainly correlated with the electron effective mass and the density-of-states.
Similar content being viewed by others
Introduction
The dimensionless thermoelectric figure-of-merit (zT) is defined as zT = S2σT/κ = S2σT/(κc + κL), where S, T, σ, κ, κc, and κL are the Seebeck coefficient, absolute temperature in Kelvin, electrical conductivity, total thermal conductivity, charge carrier thermal conductivity, and lattice thermal conductivity, respectively1,2,3,4. Enhanced zT values could be realized through adjusting the electronic structures and thermal conductivity by the doping approach5,6,7,8,9. It should be noted that zT is proportional to the square of S, indicating that improving S might be an easier way to get improved zT values, compared to regulating the other thermoelectric parameters such as σ and κ.
It is well known that σ and S can be estimated by the following formulas10,11,12,


where n, μ, kB, h, and m* are the charge carrier density, carrier mobility, Boltzmann constant, Planck constant and effective mass of the carriers, respectively. Moreover, the effective mass (m*) and carrier mobility (μ) can be deduced from the electronic band structures and density-of-states (DOS) obtained from related first-principle calculations13. In this regard, S can be predicted through performing theoretical calculations on the electronic band structures and DOS, and the estimation of σ can also be roughly achieved with considering the average scattering time as a constant14,15. Therefore, it is highly desirable to gain insight into the electronic structures to get theoretical backup for the observed experimental phenomena.
Among all the state-of-the-art high temperature p-type thermoelectric materials, the copper-ion-liquid-like Cu2−xSe and Cu2−xS compounds show high thermoelectric performance, even though there still are some issues that need to be resolved before practical applications can be considered16. It has been reported that polycrystalline Cu2−xSe and Cu2−xS bulks can achieve the highest zT values of around 1.6 and 1.7 at 1000 K17,18, which have been further improved to 1.8 and 1.919,20, respectively.
Additionally, it should be noted that the high-temperature β-Cu2−xSe and α-Cu2−xS have the same crystal structure, and both of them are superionic conductors. Besides the high crystal symmetry, these two systems also possess another important property, that of congruent melting, which means that highly dense samples can be easily fabricated by a facile melt-solidification technique.
The previous studies20,21,22 on the electronic structures for Cu2Se and Cu2S compounds indicate that the copper deficiency makes both of them intrinsic p-type conductors owing to the contributions mainly from the Cu 3d-, Se 4p-, and S 3p-states near the Fermi level (EF). These facts provide evidence that doping other elements into Cu or Se (S) sites could effectively alter their electronic structures, and consequently affect their electrical and thermal transport behaviour as well as their overall thermoelectric performance.
Generally, in order to obtain enhanced S and concurrent high σ as well as low κ values through the doping approach, the following factors should be considered when choosing dopants: (1) Dopants should have the same valence as the counterpart element, which will ensure the charge balance of the system and maintain the same crystal structure. (2) Dopants should have comparable radiuses to the counterpart element, which will result in little difference in the lattice parameters and provide good optimization of electrical and thermal transport properties.
As for the Cu2−xSe system, the doping approach, using such elements as Ag, Sb, Al, and Sn for the Cu sites23,24,25,26,27,28 and Te and I for the Se sites21,29, has been chosen to modify its electronic structures and thermal conductivity up to now. The results illustrate, however, that only a small amount doping with one of these elements could lead to limited improvements to the thermoelectric performance in this system. Generally, the lighter atoms and heavier atoms should have opposite effects on the electronic and thermal transport properties. For the substitutions on Se sites, only the heavier atoms have been investigated so far, and no enhanced thermoelectric performance was observed except at the phase transition temperatures. Therefore, it is necessary to investigate the doping effects of lighter atoms, with a smaller atomic radius, to test whether or not the doping approach could be beneficial for the further enhancement of the overall thermoelectric performance of the Cu2−xSe system.
In this work, we investigated sulphur doping effects on the thermoelectric properties of the Cu2−xSe system based on the following considerations: (1) At high temperature, α-Cu2S has the same crystal structure as β-Cu2Se, and therefore, sulphur should be very easy to substitute into the lattice and replace Se. (2) S2− has the same valence as Se2−, which should result in good electron balance in this system. (3) The unit cell size should be reduced after S substitutes for Se, which will affect the migration of copper ions and lead to varied electrical and thermal transport behaviour.
It should be noted that, for practical applications of thermoelectric materials, besides the high zT values, the thermoelectric compatibility factor (s), derived as  , is another important factor, which is crucial for the efficient operation of a high temperature thermoelectric device12,30,31,32,33. The closer the s for two n- and p-type thermoelectric materials, the higher the combined efficiency that will be achieved when they are adjoining segments in one thermoelectric device. Little information on s, however, has been reported for the Cu2−xSe or Cu2−xS based thermoelectric materials. Hence, it is meaningful to calculate the s values for the sulphur doped Cu2−xSe to gain sufficient information for their future practical applications.
, is another important factor, which is crucial for the efficient operation of a high temperature thermoelectric device12,30,31,32,33. The closer the s for two n- and p-type thermoelectric materials, the higher the combined efficiency that will be achieved when they are adjoining segments in one thermoelectric device. Little information on s, however, has been reported for the Cu2−xSe or Cu2−xS based thermoelectric materials. Hence, it is meaningful to calculate the s values for the sulphur doped Cu2−xSe to gain sufficient information for their future practical applications.
Herein, the doping effects on the thermoelectric properties of highly dense Cu1.98SxSe1−x polycrystalline bulks were investigated experimentally, in order to provide a full understanding of how the doping approach modifies the thermoelectric properties of the Cu2−xSe system. We also conducted a systematic study on the sulphur doping effects on the electronic band structures and DOS for the Cu15SxSe8−x compounds based on Density Functional Theory (DFT) calculations. The results indicate that the overall thermoelectric performance in Cu1.98SxSe1−x is strongly dependent on the sulphur doping concentration, and it is mainly correlated with the electron effective mass and DOS.
Results and Discussion
Figure 1 shows the X-ray diffraction (XRD) patterns for the fabricated Cu1.98SxSe1−x (x = 0, 0.02, 0.08, 0.16, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0) samples. The results indicate that the Cu1.98SxSe1−x samples show different crystal structures with different x values. They are single-phase and have the same monoclinic34 crystal structure as the low temperature α-phase Cu2−xSe (PDF No.: 27–113123) when x ≤ 0.16. They then become composites of low temperature cubic structured Cu1.8Se and hexagonal structured Cu2.001S when x varies in the range from 0.2 to 0.7 (0.2 ≤ x ≤ 0.7). Finally, they become single-phase orthorhombic structured Cu2S (PDF: 23–961) when x is over 0.8.
X-ray diffraction (XRD) patterns of the fabricated Cu1.98SxSe1−x (x = 0, 0.02, 0.08, 0.16, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0) samples: (a) Cu1.98Se, (b) Cu1.98S0.02Se0.98, (c) Cu1.98S0.08Se0.92, (d) Cu1.98S0.16Se0.84, (e) Cu1.98S0.2Se0.8, (f) Cu1.98S0.3Se0.7, (g) Cu1.98S0.4Se0.6, (h) Cu1.98S0.5Se0.5, (i) Cu1.98S0.6Se0.4, (j) Cu1.98S0.7Se0.3, (k) Cu1.98S0.8Se0.2, (l) Cu1.98S0.9Se0.1, (m) Cu1.98S, (n) standard XRD pattern for monoclinic structured Cu2Se, (o) standard XRD pattern for orthorhombic structured Cu2S, (p) standard XRD pattern for cubic structured Cu1.8Se, and (q) standard XRD pattern for hexagonal structured Cu2.001S. (+ data points, − calculation line, − difference line, | marker points).
Rietveld refinements were performed for all samples based on the obtained X-ray diffraction patterns and the deduced lattice parameters, and the R-factors are listed in Table S1 (Supplementary Information). The summarized phase diagram for the sulphur doping level dependence of the lattice parameters is also displayed in Fig. 2, revealing that the Cu1.98SxSe1−x (x = 0.02, 0.08, 0.16) samples have slightly reduced lattice parameters in comparison with the Cu1.98Se sample, due to the smaller radius of sulphur compared to that of selenium. In the same way, the Cu1.98S0.8Se0.2 and Cu1.98S0.9Se0.1 samples have enlarged lattice parameters in contrast to the Cu1.98S sample, owing to the larger radius of selenium.
The geometry optimized primitive cells for the Cu15SxSe8−x (x = 0, 1, 2, 4, 6, 8) compounds are displayed in Figure S2, and the lattice parameters for these compounds are summarized in Table S1 which exhibit the consistent variation trend as the obtained experimental results. The Cu15Se8 has the largest volume of 387.606 Å3, while the Cu15S8 has the smallest volume of 340.486 Å3. The Cu15SxSe8−x (x = 1, 2, 4, 6) compounds have the volume values between 352.784 and 378.266 with values decreased with increasing x.
Since both Cu2−xSe and Cu2−xS are superionic conductors, and the migration of copper ions plays important roles on the system’s electrical conductivity, the changed lattice parameters should lead to different electrical conductivity and Seebeck coefficient, which will, in turn, result in modified thermoelectric properties with different zT values. Thus, it is essential to discuss the sulphur doping effects on the thermoelectric properties of the Cu2−xSe system, as well as the selenium doping effects on the thermoelectric properties of the Cu2−xS system. Hence, we will focus on the thermoelectric properties of the single-phase Cu1.98SxSe1−x (x = 0.02, 0.08, 0.16) and Cu1.98SxSe1−x (x = 0.8, 0.9, 1.0) samples in the following part.
Figure 3a shows the temperature dependence of the electrical conductivity for the Cu1.98SxSe1−x (x = 0, 0.02, 0.08, 0.16) bulks. It indicates that compared to Cu1.98Se, the Cu1.98SxSe1−x (x = 0.02, 0.08, 0.16) samples have lower σ values over the whole measured temperature range, and the most obvious difference occurs at T = 420 K between 400 S · cm−1 for the Cu1.98S0.08Se0.92 and 900 S · cm−1 for the Cu1.98Se. It should be pointed out that this difference becomes less obvious with increasing temperature because the high temperature phases are superionic conductors.
Figure 3b displays the temperature dependence of the Seebeck coefficient for the Cu1.98SxSe1−x (x = 0, 0.02, 0.08, 0.16) bulks. It reveals that the Cu1.98SxSe1−x (x = 0.02, 0.08, 0.16) samples have larger S values than the Cu1.98Se. Specifically, among all the samples, the Cu1.98S0.08Se0.92 has the highest S values, around 275 μV · K−1 at T = 970 K, which is over 30% higher than that of the Cu1.98Se.
Figure 3c shows the temperature dependence of the thermal conductivity for the Cu1.98SxSe1−x (x = 0, 0.02, 0.08, 0.16) bulks. It indicates that both the Cu1.98S0.02Se0.98 and Cu1.98S0.08Se0.92 bulks have almost the same κ values as the Cu1.98Se, especially in the temperature range from 500 to 1000 K. The Cu1.98S0.16Se0.84, however, shows increased values over the whole temperature range from 300 to 1000 K.
The temperature dependence of the dimensionless figure-of-merit (zT) for the Cu1.98SxSe1−x (x = 0, 0.02, 0.08, 0.16) bulks is shown in Fig. 3d. It should be noted that the Cu1.98SxSe1−x (x = 0.02, 0.08, 0.16) samples show almost the same zT values as the Cu1.98Se in the temperature range from 400 to 600 K. Furthermore, they have zT values over 1.0 when T > 800 K and exhibit a peak zT at T around 950 K, with the highest value of 1.5 occurring for the Cu1.98S0.02Se0.98.
Figure 4 displays the temperature dependence of the electrical conductivity (a), Seebeck coefficient (b), total thermal conductivity (c), and dimensionless figure-of-merit (d) for the obtained Cu1.98SxSe1−x (x = 0.8, 0.9, 1.0) bulks. The results indicate that, for the high temperature cubic structured Cu2S phase, the Cu1.98SxSe1−x samples do not show a monotonic increase or decrease in their electrical conductivity compared to the Cu1.98S. The same trend is also observed for the temperature dependence of the Seebeck coefficient. Furthermore, the Cu1.98S0.8Se0.2 sample has almost the same thermal conductivity as the Cu1.98S, while the Cu1.98S0.9Se0.1 sample has much smaller thermal conductivity, with values around 0.5 W · m−1 · K−1 in the temperature range from 700 to 1000 K. This paradoxical result might be related to the characteristics of the superionic system and the larger radius as well as the heavier mass of selenium compared to sulphur. As aforementioned, the cubic structured Cu2S is a superionic conductor and it is the migration of copper ions that predominately determines its thermal conductivity. Therefore, the selenium doped samples should have higher thermal conductivity than the Cu1.98S, due to the enlarged lattice parameters evidenced from the refinements of the X-ray diffraction patterns. On the other hand, selenium is much heavier than sulphur, so the selenium doped samples should have lower thermal conductivity. As a result of these two opposite factors, the samples show the complicated and varying trend that is observed for the thermal conductivity.
The temperature dependence of the dimensionless figure-of-merit shown in Fig. 4d reveals that the selenium doping does not improve the overall thermoelectric performance of the Cu1.98S system, with the Cu1.98S having the highest zT values around 0.86 at 850 K among all the orthorhombic structured samples, even though certain thermoelectric parameter is enhanced. This observation is in good agreement with our previous reports on the tellurium and iodine doped Cu2−xSe system21, which provides further evidence of the distinctiveness of superionic thermoelectric materials in comparison with the conventional thermoelectric materials.
Figure 5 shows a summarized phase diagram for the sulphur doping level dependence of the dimensionless figure-of-merit for the obtained Cu1.98SxSe1−x (x = 0, 0.02, 0.08, 0.16, 0.8, 0.9, 1.0) samples. It indicates that the zT values are first reduced as x increases from 0 to 0.16, and then they are enhanced as x increases from 0.8 to 1.0, which agrees very well with our previous theoretical and experimental discussions on sulphur (selenium) doping effects on the superionic Cu2−xSe(S) system.
For the practical applications of thermoelectric materials, besides the high zT values that are needed, the thermoelectric compatibility factor, s, is another important factor that needs to be considered when designing an efficient thermoelectric generator12,32,35. The maximum efficiency of a thermoelectric generator will be decreased greatly if the compatibility factors for the segments of n- and p-type thermoelectric materials differ from each other by a factor larger than two. Therefore, in order to discover the best applicable temperature range and best matching material for a certain thermoelectric material, the calculation of s is quite essential.
Figure 6 shows the temperature dependence of the thermoelectric compatibility factor for the Cu1.98SxSe1−x (x = 0, 0.02, 0.08, 0.16, 0.8, 0.9, 1.0) bulks. It indicates that all the samples exhibit stable s values with small fluctuations over a large temperature range from 700 to 1000 K. In the measured temperature range, average s values of around 3.85, 2.92, 2.14, and 2.56 were achieved for the Cu2Se-phase Cu1.98Se, Cu1.98S0.02Se0.98, Cu1.98S0.08Se0.92, and Cu1.98S0.16Se0.84 samples, respectively. Furthermore, the Cu2S-phase samples Cu1.98S0.8Se0.2, Cu1.98S0.9Se0.1, and Cu1.98S exhibit average s values of ~0.72, 0.87, and 0.93 in the temperature range from 700 to 1000 K, respectively.
For comparison purposes, the s values for some well-known n-type thermoelectric materials are also provided in Fig. 6. It indicates that polycrystalline SiGe, PbTe, La2Te3, and CoSb3 bulks show s of around 1.37, 1.90, 2.14, and 2.31, respectively. It should be noted that these are very close to the s values of the Cu1.98SxSe1−x (x = 0, 0.02, 0.08, 0.16, 0.8, 0.9, 1.0) bulks, with the differences less than a factor of 2 in the temperature range from 700 to 1000 K. Therefore, a relatively high efficiency could be gained from their coupled thermoelectric modules, which is gratifying for their future practical applications.
As aforementioned in the introduction part, the electrical conductivity and Seebeck coefficient is related to the charge carrier density, carrier mobility, and effective mass, which can be deduced from the calculated DOS and electronic band structures. Therefore, in order to fundamentally understand the strange sulphur doping effects on the thermoelectric properties of the Cu1.98SxSe1−x system, it is much necessary to do some first-principles calculations on the system’s electronic band structures and DOS.
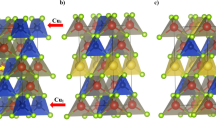
Figure 7 shows a perspective view of the crystal structures for the cubic structured Cu2Se(S), as well as the ideal versions of the unit cell22 and the primitive cell. It indicates that, in the ideal version of the unit cell for the cubic structured Cu2Se(S) with space group of  , the selenium (sulphur) atoms form a face-centred cubic (fcc) sub-lattice, and the copper atoms occupy the tetrahedral interstitial positions. It should, however, be noted that β-Cu2Se and α-Cu2S have been reported to be superionic conductors, in which the copper ions behave like a liquid17,19,36,37,38, and they are kinetically disordered throughout the whole structure.
, the selenium (sulphur) atoms form a face-centred cubic (fcc) sub-lattice, and the copper atoms occupy the tetrahedral interstitial positions. It should, however, be noted that β-Cu2Se and α-Cu2S have been reported to be superionic conductors, in which the copper ions behave like a liquid17,19,36,37,38, and they are kinetically disordered throughout the whole structure.
Figure 8 shows the calculated total and partial DOS for the Cu15SxSe8−x (x = 1, 2, 4, 6) compounds. The sulphur doping level dependence of the total and partial DOS at EF for the Cu15SxSe8−x (x = 0, 1, 2, 4, 6, 8) compounds is displayed in Fig. 9. The results indicate that the DOS at EF consists of the contributions from the Cu 3d-states, Se 4p-states, and S 3p-states of the Cu15SxSe8−x compounds, with the Cu 3d-states predominately determining the total DOS at EF. In addition, the partial DOS for the S atoms obviously increases with increasing x, while the partial DOS for the Se atoms shows the opposite trend, decreasing with increasing x. The total DOS exhibits the same non-monotonic tendency as the partial DOS for the Cu atoms, further indicating that the DOS at EF for this system is mainly determined by the copper atoms rather than the S or Se atoms. In summary, the total DOS firstly increases slightly, then clearly decreases, with the Cu15S6Se2 having the highest DOS among all the compounds, ~6.29856 states/eV/f.u.
(a,b,c,d) total and partial DOS for the Cu15S1Se7. (e,f,g,h) total and partial DOS for the Cu15S2Se6. (i,j,k,l) total and partial DOS for the Cu15S4Se4. (m,n,o,p) total and partial DOS for the Cu15S6Se2. The total DOS for the Cu, Se, and S atoms are displayed, and the partial DOS for the 4s-, 3p-, and 3d-states of Cu, the 4s- and 4p-states of Se, and the 3s- and 3p-states of S are also presented. The vertical lines mark the position of the Fermi level (EF).
The effective mass can be directly deduced from the calculated DOS based on some references13,39. Figure 10 shows the deduced effective mass for the Cu15SxSe8−x (x = 0, 1, 2, 4, 6, 8) compounds. It indicates that, similar to the DOS, the sulphur doping also has a non-monotonic effect on the effective mass. It firstly has positive effects and gives the Cu15S4Se4 its highest m*, with a value of ~1.151 me. It then shows negative effects and results in decreased m* values as the sulphur doping level increases.
According to Equations 1–4 and previous discussions on σ, S, n and μ, we know that both σ and S are linked to the m* and the DOS. Specifically, σ is proportional to the carrier density (n) and inversely proportional to m*. S, however, is proportional to m* and  . Therefore, based on the obtained information on the DOS and m*, we can anticipate the variation trends for the values of S. σ values can also be roughly estimated with disregarding the average scattering time. Hence, we can predict that the cubic structured sulphur doped Cu2−xSe compounds should exhibit non-monotonic variation in σ and S with increasing sulphur concentration according to the calculated DOS and m* using the DFT method.
. Therefore, based on the obtained information on the DOS and m*, we can anticipate the variation trends for the values of S. σ values can also be roughly estimated with disregarding the average scattering time. Hence, we can predict that the cubic structured sulphur doped Cu2−xSe compounds should exhibit non-monotonic variation in σ and S with increasing sulphur concentration according to the calculated DOS and m* using the DFT method.
It should be pointed out that, for the monoclinic structured Cu2Se phased Cu1.98SxSe1−x samples, the observed sulphur doping level dependence of the electrical conductivity and the Seebeck coefficient is in good agreement with the theoretical predictions. The DOS is enhanced as the sulphur doping level increases, while the effective mass is reduced as the doping level increases. Additionally, σ and S are proportional to  and m*, respectively. Therefore, enhanced electrical conductivity and reduced Seebeck coefficient can be obtained when the contribution from the DOS is stronger than that from m*. When the contribution of the effective mass is stronger, decreased electrical conductivity and increased Seebeck coefficient will be achieved.
and m*, respectively. Therefore, enhanced electrical conductivity and reduced Seebeck coefficient can be obtained when the contribution from the DOS is stronger than that from m*. When the contribution of the effective mass is stronger, decreased electrical conductivity and increased Seebeck coefficient will be achieved.
In summary, the Cu1.98SxSe1−x compounds have the same crystal structure as monoclinic structured Cu2Se when x ≤ 0.16, become composites of cubic structured Cu1.8Se and hexagonal structured Cu2.001S when 0.2 ≤ x ≤ 0.7, and finally have the same crystal structure as orthorhombic structured Cu2S when 0.8 ≤ x ≤ 1.0. The overall thermoelectric performance of the Cu1.98SxSe1−x compounds is mainly correlated with the electron effective mass and the density of states, with the zT values first increasing and then decreasing. Additionally, all the samples show stable thermoelectric compatibility factors over a broad temperature range from 700 to 1000 K, which could greatly benefit their practical applications. DFT calculations indicate that sulphur doping has non-monotonic effects on the DOS and m*, with the Cu15S6Se2 and Cu15S4Se4 having the highest DOS value, ~0.69 states/eV/f.u., and the highest m*, ~0.336 me, respectively.
Methods
Sample preparation
Polycrystalline Cu1.98SxSe1−x pellets were synthesized by a conventional solid-state method. Mixtures of Cu, S, and Se powders in the molar ratios of 1.98 : x : 1 − x (x = 0, 0.02, 0.08, 0.16, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7 0.8, 0.9, 1.0) were pressed into pellets and sealed in evacuated quartz tubes, then heated to 873 K for 1–5 hours with a heating rate of 5 K/min, followed by a furnace cooling to room temperature. Finally, the as-sintered pellets were used in a melt-solidification approach to achieve highly dense polycrystalline bulks, which has been described in detail in our previous work19,21. The obtained polycrystalline bulks were then shaped into round disks and rectangular bulks for electrical conductivity and thermal diffusivity measurements, respectively.
Measurements
X-ray diffraction (XRD) patterns were collected on a GBC MMA system using Cu Kα radiation. The electrical conductivity and Seebeck coefficient were measured simultaneously in a helium atmosphere from 300 to 973 K using an RZ2001i system. The thermal diffusivity (D) was measured by the laser flash method (LINSEIS LFA 1000), and the specific heat (Cp) was determined by differential scanning calorimetry (NETZSCH DSC 204F1). The sample density (dd) was determined by the sample mass divided by volume, and the thermal conductivity (κ) was calculated according to κ = D × Cp × dd.
Calculations
The calculations of electronic band structures, and total and partial DOS were performed based on the DFT method, implemented by the CASTEP package40 with the generalized gradient approximation (GGA)41. The calculations were parameterized by the Perdew-Burke-Ernzerhof (PBE)42 and ultra-soft pseudo-potentials. The plane wave cut-off energy was set at 400 eV. For Cu2Se and Cu2S, a primitive cell with the Brillouin zone path of ΓXWLΓK (Fig. S1 in Electronic Supplementary Information) was employed for the band structure calculations. For the Cu15SxSe8−x (x = 0, 1, 2, 4, 6, 8), geometry optimizations were performed on a 2 × 2 × 2 supercell, with one copper atom deleted, of the Cu2Se primitive cell. Then, the Brillouin zone path of ΓXWLΓK and a k-point set of 15 × 15 × 15 for the supercell were used to calculate the electronic band structures, and the total and partial DOS, respectively.
Additional Information
How to cite this article: Zhao, L. et al. Improvement of thermoelectric properties and their correlations with electron effective mass in Cu1.98SxSe1−x. Sci. Rep. 7, 40436; doi: 10.1038/srep40436 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Zeier, W. G. et al. Phonon scattering through a local anisotropic structural disorder in the thermoelectric solid solution Cu2Zn1−x Fe x GeSe4 . J. Am. Chem. Soc. 135, 726–732, doi: 10.1021/ja308627v (2013).
Liu, W., Yan, X., Chen, G. & Ren, Z. Recent advances in thermoelectric nanocomposites. Nano Energy 1, 42–56, doi: 10.1016/j.nanoen.2011.10.001 (2012).
Jood, P. et al. Al-doped zinc oxide nanocomposites with enhanced thermoelectric properties. Nano Lett. 11, 4337–4342, doi: 10.1021/nl202439h (2011).
Zhang, Y., Wang, X. L., Yeoh, W. K., Zheng, R. K. & Zhang, C. Electrical and thermoelectric properties of single-wall carbon nanotube doped Bi2Te3 . Appl. Phys. Lett. 101, 031909, doi: 10.1063/1.4737898 (2012).
Pei, Y. et al. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 473, 66–69, doi: 10.1038/nature09996 (2011).
Pei, Y. et al. Optimum Carrier Concentration in n-Type PbTe Thermoelectrics. Adv. Energy Mater. 4, 1400486, doi: 10.1002/aenm.201400486 (2014).
Yamini, S. A. et al. Chemical composition tuning in quaternary p-type Pb-chalcogenides--a promising strategy for enhanced thermoelectric performance. Phys. Chem. Chem. Phys. 16, 1835–1840, doi: 10.1039/c3cp54493a (2014).
Pei, Y., Heinz, N. A. & Snyder, G. J. Alloying to increase the band gap for improving thermoelectric properties of Ag2Te. J. Mater. Chem. 21, 18256, doi: 10.1039/c1jm13888j (2011).
Wang, H., Pei, Y., LaLonde, A. D. & Snyder, G. J. Heavily doped p-type PbSe with high thermoelectric performance: an alternative for PbTe. Adv. Mater. 23, 1366–13370, doi: 10.1002/adma.201004200 (2011).
Cutler, M., Leavy, J. & Fitzpatrick, R. Electronic Transport in Semimetallic Cerium Sulfide. Phys. Rev. 133, A1143–A1152, doi: 10.1103/PhysRev.133.A1143 (1964).
Bulusu, A. & Walker, D. G. Review of electronic transport models for thermoelectric materials. Superlattice Microst. 44, 1–36, doi: 10.1016/j.spmi.2008.02.008 (2008).
Snyder, G. J. & Toberer, E. S. Complex thermoelectric materials. Nat. Mater. 7, 10 (2008).
Lange, B., Freysoldt, C. & Neugebauer, J. Native and hydrogen-containing point defects in Mg3N2: A density functional theory study. Phys. Rev. B 81, 224109, doi: 10.1103/PhysRevB.81.224109 (2010).
Parker, D., Chen, X. & Singh, D. J. High three dimensional thermoelectric performance from low dimensional bands. Phys. Rev. Lett. 110, 146601 (2013).
Sun, B. Z., Ma, Z., He, C. & Kechen, W. Anisotropic thermoelectric properties of layered compounds in SnX2 (X = S, Se): A promising thermoelectric material. Phys. Chem. Chem. Phys., doi: 10.1039/c5cp03700j (2015).
Dennler, G. et al. Are Binary Copper Sulfides/Selenides Really New and Promising Thermoelectric Materials? Adv. Energy Mater. 4, 1301581, doi: 10.1002/aenm.201301581 (2014).
Liu, H. et al. Copper ion liquid-like thermoelectrics. Nat. Mater. 11, 422–425, doi: 10.1038/nmat3273 (2012).
He, Y. et al. High thermoelectric performance in non-toxic earth-abundant copper sulfide. Adv. Mater. 26, 3974–3978, doi: 10.1002/adma.201400515 (2014).
Zhao, L. et al. Superior intrinsic thermoelectric performance with zT of 1.8 in single-crystal and melt-quenched highly dense Cu2−x Se bulks. Sci. Rep. 5, 7671, doi: 10.1038/srep07671 (2015).
Zhao, L. et al. High thermoelectric and mechanical performance in highly dense Cu2−x S bulks prepared by a melt-solidification technique. J. Mater. Chem. A 3, 9432–9437, doi: 10.1039/c5ta01667c (2015).
Zhao, L. et al. The Effects of Te2− and I− Substitutions on the Electronic Structures, Thermoelectric Performance, and Hardness in Melt-Quenched Highly Dense Cu2−x Se. Advanced Electronic Materials 1, 1400015, doi: 10.1002/aelm.201400015 (2015).
Rasander, M., Bergqvist, L. & Delin, A. Density functional theory study of the electronic structure of fluorite Cu2Se. J. Phys. Condens. Matter. 25, 125503, doi: 10.1088/0953-8984/25/12/125503 (2013).
Ballikaya, S., Chi, H., Salvador, J. R. & Uher, C. Thermoelectric properties of Ag-doped Cu2Se and Cu2Te. J. Mater. Chem. A 1, 12478, doi: 10.1039/c3ta12508d (2013).
Ibáñez, M. et al. Colloidal synthesis and thermoelectric properties of Cu2SnSe3 nanocrystals. Journal of Materials Chemistry A 1, 1421, doi: 10.1039/c2ta00419d (2013).
Suzumura, A., Watanabe, M., Nagasako, N. & Asahi, R. Improvement in Thermoelectric Properties of Se-Free Cu3SbS4 Compound. J Electron Mater 43, 2356–2361, doi: 10.1007/s11664-014-3064-y (2014).
Zhong, B. et al. High superionic conduction arising from aligned large lamellae and large figure of merit in bulk Cu1.94Al0.02Se. Appl. Phys. Lett. 105, 123902, doi: 10.1063/1.4896520 (2014).
Fan, J. et al. Crystal Structure and Physical Properties of Ternary Phases around the Composition Cu5Sn2Se7 with Tetrahedral Coordination of Atoms. Chem Mater 26, 5244–5251, doi: 10.1021/cm501899q (2014).
Tyagi, K. et al. Thermoelectric and mechanical properties of spark plasma sintered Cu3SbSe3 and Cu3SbSe4: Promising thermoelectric materials. Appl. Phys. Lett. 105, 261902, doi: 10.1063/1.4904996 (2014).
Liu, H. et al. Ultrahigh thermoelectric performance by electron and phonon critical scattering in Cu2Se1−x Ix . Adv. Mater. 25, 6607–6612, doi: 10.1002/adma.201302660 (2013).
Snyder, G. J. Application of the compatibility factor to the design of segmented and cascaded thermoelectric generators. Appl. Phys. Lett. 84, 2436, doi: 10.1063/1.1689396 (2004).
Snyder, G. & Ursell, T. Thermoelectric efficiency and compatibility. Phys. Rev. Lett. 91, 148301, doi: 10.1103/PhysRevLett.91.148301 (2003).
Snyder, G. J. & Caillat, T. Using the compatibility factor to design high efficiency segmented thermoelectric generators. MRS Proceedings 793, 37–42 (2003).
Bhardwaj, A., Chauhan, N. S. & Misra, D. K. Significantly enhanced thermoelectric figure of merit of p-type Mg3Sb2-based Zintl phase compound via nanostructuring employing high energy mechanical milling coupled with spark plasma sintering. J. Mater. Chem. A 3, 10777, doi: 10.1039/c5ta02155c (2015).
Gulay, L., Daszkiewicz, M., Strok, O. & Pietraszko, A. Crystal structure of Cu2Se. Chem. Met. Alloys 4, 200–205 (2011).
Ursell, T. S. & Snyder, G. J. Compatibility of Segmented Thermoelectric Generators. Twenty-First International Conference on Thermoelectrics, 412–417 (2002).
Oliveria, M., McMullan, R. K. & Wuensch, B. J. Single crystal neutron diffraction analysis of the cation distribution in the high-temerpature phases a-Cu2-xS, a-Cu2-xSe, AND a-Ag2Se. Solid State Ion. 28-30, 1332–1337 (1988).
Heyding, R. D. & Murry, R. M. The crystal structures of Cu1.8Se, Cu3Se2, α- and γ-CuSe, CuSe2 and CuSe2 II. Can. J. Chem. 54, 841–848 (1976).
Lu, P. et al. Multiformity and fluctuation of Cu ordering in Cu2Se thermoelectric materials. J. mater. Chem. A 3, 6901–6908, doi: 10.1039/C4TA07100J (2015).
Jaworski, C. M., Wiendlocha, B., Jovovic, V. & Heremans, J. P. Combining alloy scattering of phonons and resonant electronic levels to reach a high thermoelectric figure of merit in PbTeSe and PbTeS alloys. Energy Environ. Sci. 4, 4155, doi: 10.1039/c1ee01895g (2011).
Clark, S. J. et al. First principles methods using CASTEP. Z. Kristallogr. 220, 567–570 (2005).
Hohenberg, P. Inhomogeneous electron gas. Phys. Rev. 136, B864–B871, doi: 10.1103/PhysRev.136.B864 (1964).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Acknowledgements
X.L.W. acknowledges the support for this work from the Australian Research Council (ARC) through an ARC Discovery Project (DP 130102956) and an ARC Professorial Future Fellowship project (FT 130100778) and an ARC LIEF project (LE120100069, X.L.W). L.L.Z. is grateful for the support from the Fundamental Research Funds of Shandong University (11160076614087) and the Natural Science Foundation of Shandong Province (ZR2013EMM014 and ZR2013EMM018). J.W. is grateful for the support from the Fundamental Research Funds of Shandong University (31370076614103). The authors would like to thank Prof. G. Jeffrey Snyder (Northwestern University) for helpful discussions on the calculations and experimental results. The authors would also thank Dr. Tania Silver for polishing the English of the manuscript.
Author information
Authors and Affiliations
Contributions
X.L.W. and L.L.Z designed the experiments and theoretical calculations. L.L.Z. and J.W. fabricated samples and performed the XRD and measurements. L.L.Z., F.Y.F., F.N.W., C.L.W. and J.C.L. did the theoretical calculations. L.L.Z., F.Y.F., J.W., F.N.W., C.L.W., J.C.L., J.Y.W., Z.X.C., S.X.D., and X.L.W. analyzed and discussed the data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhao, L., Fei, F., Wang, J. et al. Improvement of thermoelectric properties and their correlations with electron effective mass in Cu1.98SxSe1−x. Sci Rep 7, 40436 (2017). https://doi.org/10.1038/srep40436
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40436
- Springer Nature Limited
This article is cited by
-
The thermoelectric properties of Cu2S are enhanced by doping tin with hydrothermal method
Journal of Materials Science: Materials in Electronics (2024)
-
Transport phenomena in superionic Na х Cu2 − х S (х = 0.05; 0.1; 0.15; 0.2) compounds
Ionics (2018)
-
Optimum electronic structures for high thermoelectric figure of merit within several isotropic elastic scattering models
Scientific Reports (2017)