Abstract
Delayed gastric emptying and impaired gastric accommodation (decreased gastric compliance) play important roles in functional dyspepsia (FD). Here we screen for a clinically used drug with an ability to improve delayed gastric emptying in rats. Oral administration of aldioxa (dihydroxyaluminum allantoinate) partially improved clonidine- or restraint stress-induced delayed gastric emptying. Administration of allantoin, but not aluminium hydroxide, restored the gastric emptying. Both aldioxa and allantoin inhibited clonidine binding to the α-2 adrenergic receptor, suggesting that antagonistic activity of the allantoin moiety of aldioxa on this receptor is involved in the restoration of gastric emptying activity. Aldioxa or aluminium hydroxide but not allantoin restored gastric compliance with restraint stress, suggesting that aluminium hydroxide moiety is involved in this restoration. We propose that aldioxa is a candidate drug for FD, because its safety in humans has already been confirmed and its ameliorating effect on both of delayed gastric emptying and impaired gastric compliance are confirmed here.
Similar content being viewed by others
Introduction
Functional dyspepsia (FD) is a disease defined as persistent or recurrent postprandial upperabdominal discomfort and epigastric pain in the absence of any organic, systemic, or metabolic diseases. The prevalence of dyspepsia in the general population is remarkably high (15–20%) and the majority of these patients is believed to have FD1. Although FD is not a life-threatening disease, it markedly reduces patients’ quality of life and places a significant economic burden on the healthcare system2. However, protocols to treat FD, including pharmacotherapy, have not been fully efficacious, with approximately 50% of FD patients remaining symptomatic over a 5-year follow-up period1.
According to Rome III criteria, FD is categorized into postprandial-distress syndrome (PDS) and epigastric pain syndrome (EPS), based on the predominant symptoms (postprandial fullness/early satiation and epigastric pain/burning, respectively)3,4. Delayed gastric emptying and impaired gastric accommodation to a meal or visceral hypersensitivity to gastric distension have been suggested to be involved in the pathogenesis of PDS or EPS, respectively5.
While the mechanism of delayed gastric emptying in FD patients has been widely investigated over the past two decades, no single definitive reason for the condition has been identified. Thus, a combination of physiologic, genetic, environmental and psychological factors are supposed to cause delayed gastric emptying seen in FD patients. Various endogenous molecules, including hypothalamic-pituitary-adrenal axis system-related molecules (such as corticotropin-releasing hormone and corticosterone), ghrelin, serotonin (5-HT) and dopamine affect gastric emptying and were suggested to be involved in delayed gastric emptying in FD patients6,7. Consequently, these molecules and their receptors have received considerable attention as targets for anti-FD drugs. For example, mosapride citrate (mosapride), cisapride monohydrate (cisapride) and tegaserod are 5-HT4 receptor agonists, while domperidone and itopride hydrochloride (itopride) are dopamine 2 (D2) receptor antagonists; all of these drugs are currently used or had been used to treat FD patients in some countries8. However, pharmacological therapy by these drugs for FD patients has resulted in outcomes that have been unsatisfactory9. Furthermore, since these endogenous molecules also regulate other physiological functions, especially heart function, the use of these drugs is restricted clinically due to adverse effects, such as cardiotoxicity. In fact, cisapride has been withdrawn from the US market due to its cardiotoxic properties (long QT syndrome)10. Thus, new target molecules for anti-FD drugs need be identified, one approach to follow is the phenotype screening of compounds that could stimulate gastric emptying in animals.
Acetylcholine is also a key positive regulator for gastric emptying and it was reported that dysfunction of the vagus nerve and a resulting decreased level of acetylcholine release from nerve terminals are involved in the delayed gastric emptying seen in FD patients11,12. Furthermore, acotiamide hydrochloride (acotiamide), the first drug approved to treat FD in Japan, is an acetylcholinesterase inhibitor and was suggested to stimulate gastric emptying by increasing acetylcholine levels in vagus nerve terminals13. Various receptor types, such as the α-2 adrenergic, D2 and 5-HT4 receptors, affect the gastric emptying, at least partially, through regulation of this acetylcholine release14,15. Thus, drugs that affect the acetylcholine release might be good candidates as anti-FD drugs.
Gastric accommodation provides temporary storage for ingested food prior to its transition into the intestine. This process consists of a reduction in gastric tone and an increase in compliance in response to food intake, allowing an increased fundic volume without any rise in intragastric pressure16. In addition to delayed gastric emptying, impaired gastric accommodation plays an important role in the pathogenesis of FD, particularly PDS5,17. The importance of impaired gastric accommodation in the pathogenesis of FD was further suggested by a recent finding that acotiamide stimulates gastric accommodation18, although the mechanism is unknown.
The number of drugs reaching the marketplace each year is decreasing, mainly due to the unexpected adverse effects of potential drugs being revealed in clinical trials. Thus, we have proposed a new strategy for drug discovery and development (drug re-positioning strategy), which focuses on the use of existing medicines for alternative indications19. In this strategy, compounds with clinically beneficial pharmacological activity are screened from a library of medicines already in clinical use to be developed for new indications. The advantage of this strategy is that there is a decreased risk for unexpected adverse effects in humans because the safety aspects of these drugs have already been well characterized19.
In the present study, we undertook both strategies of phenotypic screening in animals and drug re-positioning to search candidate drugs for FD. The drug aldioxa, a medicine used clinically for many years to treat gastric ulcers and gastritis, was identified20,21. Orally administered aldioxa partially restored gastric emptying that was impaired by the administration of clonidine hydrochloride (clonidine) or in response to restraint stress. Our results suggest that aldioxa restores delayed gastric emptying via its capacity to antagonise α-2 adrenergic receptor activity. We also found that aldioxa could increase gastric compliance in rats subjected to wrap restraint stress. Based on these results, we propose that aldioxa could serve as a candidate drug for FD given that its safety in humans has previously been confirmed.
Results
Effect of aldioxa on clonidine-induced delayed gastric emptying
From a group of 209 drugs already in clinical use, we screened for compounds able to suppress clonidine-induced delayed gastric emptying; each drug was administered intraperitoneally to ICR mice at a dose 30 times higher than its clinical dose, then clonidine was administered and the gastric emptying was measured by the phenol red marker method. In this manner, we selected aldioxa based on the level of restoration of gastric emptying and on clinical data attesting to its efficacy and safety. Aldioxa has been used clinically in Japan for many years to treat gastric ulcers and gastritis20,21.
To begin with, we examined the effect of oral administration of aldioxa on clonidine-induced delayed gastric emptying. As shown in Fig. 1a, administration of clonidine delayed gastric emptying and oral pre-administration of aldioxa partially restored the gastric emptying in a dose-dependent manner. The significant restoration was observed at both 200 and 670 mg kg−1 and the extent of restoration was similar between these doses (Fig. 1a). Restorative activity was also observed in mice treated with cisapride (Fig. 1a) as described previously22.
Effect of aldioxa on clonidine-induced delayed gastric emptying.
Mice fasted for 18 h were orally administered indicated dose (a) or 200 mg kg−1 (b,c) of aldioxa, indicated dose of cisapride (a) or vehicle (1% methylcellulose) (a–c). Fifty-five (a) or forty-five (b,c) minutes after each drug administration, delayed gastric emptying was induced by the subcutaneous administration of clonidine (100 (a) or 30 (b,c) μg kg−1). Control mice were subcutaneously administered saline (a–c). Gastric emptying was measured using the phenol red method (a) or the [13C]-labeled acetic acid breath test (b,c) and gastric emptying (a), Δ13CO2 (%) (b) and T1/2 (c) were calculated as described in the Methods. Values are mean ± s.e.m. *P < 0.05; **P < 0.01 (Tukey test). Experiments were replicated at least two times.
Gastric emptying in mice was also measured by the [13C]-labeled acetic acid breath test. Orally administered [13C]-labeled acetic acid is rapidly absorbed in the small intestine (but not in the stomach) and metabolized to 13CO2. Thus, by monitoring the time course 13CO2 content in the expired air, we can evaluate gastric emptying23. As shown in Fig. 1b, administration of clonidine decreased the peak level of Δ13CO2 (%; relative amount of 13CO2 in expired air) and delayed the time to peak, showing that clonidine delays gastric emptying. Pre-administration of aldioxa returned the Δ13CO2 (%) profile towards that of control mice (without clonidine treatment) (Fig. 1b), suggesting that aldioxa partially improves clonidine-induced delayed gastric emptying. Gastric emptying ability can be estimated from T1/2 of the AUC90 min of Δ13CO2 (%) (time when half of AUC90 min of Δ13CO2 (%) had been reached)23. As shown in Fig. 1c, administration of clonidine prolonged the T1/2 value, which was partially returned to control levels by pre-administration of animals with aldioxa.
Relationship between gastroprotective effect and ameliorating effect on delayed gastric emptying of aldioxa
Aldioxa is the generic name for the metal complex, dihydroxyaluminum allantoinate, which is hydrolyzed to allantoin and aluminium hydrate at the gastric mucosa24,25. The molecular mechanism governing the gastroprotective effect of aldioxa is unclear; however, it was suggested that both the direct coating of the gastric mucosa with aluminium hydrate and the anti-inflammatory effect of allantoin are involved26,27. To understand the relationship between aldioxa’s gastroprotective effect and its stimulatory effect on gastric emptying, we compared its dose-response profiles on these effects. As shown in Fig. 2a, compared to its actions on delayed gastric emptying (Fig. 1a), a much higher dose of aldioxa (1,600 mg kg−1) was required to suppress indomethacin-induced gastric lesions. We also examined the effects of other gastroprotective drugs on clonidine-induced delayed gastric emptying. Neither geranylgeranylacetone (GGA) nor sucralfate affected clonidine-induced delayed gastric emptying (Fig. 2b) at doses that could suppress indomethacin-induced gastric lesions (Fig. 2a).
Effect of gastroprotective drugs on clonidine-induced delayed gastric emptying.
Mice fasted for 18 h were orally administered indicated dose of aldioxa, geranylgeranylacetone (GGA) or sucralfate. One hour later, 20 mg kg−1 of indomethacin was orally administered and 8 h later, stomachs were removed and gastric mucosal lesions were measured (a). The effect of GGA or sucralfate at indicated dose on clonidine-induced delayed gastric emptying was examined using the phenol red method as described in the legend of Fig. 1 (b). Values are mean ± s.e.m. *P < 0.05; **P < 0.01 (Tukey test). n.s., not significant. Experiments were replicated at least two times.
We also separately examined the effect of allantoin or aluminium hydrate on clonidine-induced delayed gastric emptying. As shown in Fig. 3, allantoin but not aluminium hydrate partially improved clonidine-induced delayed gastric emptying at an equivalent dose to that of aldioxa (with respect to molecular number, 200 mg kg−1 of aldioxa corresponds to 145 or 72 mg kg−1 of allantoin or aluminium hydrate, respectively). The results in Figs 2 and 3 thus suggest that aldioxa achieves its stimulatory effect on gastric emptying independently of its gastroprotective effect and that the allantoin moiety of aldioxa is important for improved emptying.
Effect of allantoin or aluminium hydrate on clonidine-induced delayed gastric emptying.
Mice fasted for 18 h were orally administered aldioxa (200 mg kg−1), allantoin (145 mg kg−1), aluminium hydrate (Al(OH)3) (72 mg kg−1) or vehicle (1% methylcellulose). Clonidine-induced delayed gastric emptying was induced and measured using the phenol red method as described in the legend of Fig. 1. Values are mean ± s.e.m. *P < 0.05 (Tukey test). n.s., not significant. Experiments were replicated at least two times.
Effect of aldioxa on restraint stress-induced delayed gastric emptying and normal gastric emptying
Restraint stress-induced delayed gastric emptying has been used as an alternative animal model of FD28, prompting us to examine here the efficacy of aldioxa in this model. As shown in Fig. 4a, the phenol red method revealed that mice subjected to restraint stress showed delayed gastric emptying, which was partially suppressed by the pre-administration of aldioxa or mosapride to animals. Similar results were observed with the [13C]-labeled acetic acid breath test (Fig. 4b,c).
Effect of aldioxa on restraint stress-induced delayed gastric emptying and normal gastric emptying.
Mice fasted for 18 h were orally administered aldioxa (200 mg kg−1) (a–d), mosapride (7.7 mg kg−1) (a–d), cisapride (5 mg kg−1) (d) or vehicle (1% methylcellulose) (a–d) and delayed gastric emptying was induced by restraint stress (RS) (a–c). One hour after the administration of each test compound, gastric emptying was measured using the phenol red method (a,d) or the [13C]-labeled acetic acid breath test (b,c) as described in the legend of Fig. 1. Values are mean ± s.e.m. *P < 0.05; **P < 0.01 (Tukey test). n.s., not significant. Experiments were replicated at least two times.
We also examined the effect of orally administered aldioxa on the basal level of gastric emptying in intact mice (without restraint stress or clonidine treatment). Administration of cisapride or mosapride accelerated normal gastric emptying in intact mice as described previously28,29. However, aldioxa did not affect normal gastric emptying (Fig. 4d).
Mechanism for the stimulatory effect of aldioxa on gastric emptying
As described in the introduction, a number of molecular targets of drugs that could restore gastric emptying have been proposed, particularly those involving the α-2 adrenergic, D2 and 5-HT4 receptor types. We examined here the involvement of these receptors in the stimulatory effect of aldioxa on gastric emptying. To test the involvement of the 5-HT4 receptor, we examined the effect of a selective 5-HT4 receptor antagonist (GR113808)30 on the stimulatory effect of aldioxa on gastric emptying. As shown in Fig. 5a, aldioxa suppressed clonidine-induced delayed gastric emptying even in the presence of GR113808. On the other hand, cisapride (a 5-HT4 agonist) did not affect clonidine-induced delayed gastric emptying in the presence of GR113808 (Fig. 5a).
Stimulatory effect of aldioxa on gastric emptying was independent of 5-HT4 and D2 receptors.
Mice fasted for 18 h were orally administered aldioxa (200 mg kg−1) (a,b), cisapride (5 mg kg−1) (a), itopride (200 mg kg−1) (b) or vehicle (1% methylcellulose) (a,b). GR113808 (10 mg kg−1), a selective 5-HT4 antagonist, was administered 5 min before each drug administration (a). Forty-five (a) or thirty (b) minutes after each drug administration, delayed gastric emptying was induced by administration of clonidine (100 μg kg−1) (a) or apomorphine (5 mg kg−1) (b) and examined using the phenol red method as described in the legend of Fig. 1. Values are mean ± s.e.m. *P < 0.05; **P < 0.01 (Tukey test). n.s., not significant. Experiments were replicated at least two times.
To test the involvement of the D2 receptor, we next examined the effect of aldioxa on (R)-apomorphine hydrochloride hemihydrate (apomorphine, a dopamine receptor agonist)-induced delayed gastric emptying. As shown in Fig. 5b, aldioxa had no effect on this treatment. On the other hand, administration of itopride (a D2 receptor antagonist) suppressed the apomorphine-induced delayed gastric emptying (Fig. 5b). These results suggest that aldioxa restores gastric emptying independently of D2 and 5-HT4 receptor involvement.
Next, we tested involvement of the α-2 adrenergic receptor. Since activation of this receptor is coupled with a decrease in the intracellular level of cAMP31, we examined the effect of aldioxa on this receptor by monitoring intracellular cAMP levels. As shown in Fig. 6a, treatment of human neuroblastoma (SH-SY5Y) cells with clonidine decreased intracellular cAMP levels in a manner that was suppressed by simultaneous treatment with aldioxa or yohimbine hydrochloride (yohimbine, an α-2 adrenergic receptor antagonist). We also found that allantoin but not aluminium hydrate restored intracellular cAMP levels seen in the presence of clonidine and that the level of restoration was similar to that observed with aldioxa (Fig. 6b). These results suggest that aldioxa has an antagonizing effect on α-2 adrenergic receptor activation via its allantoin moiety.
Involvement of α-2 adrenergic receptor in stimulatory effect of aldioxa on gastric emptying.
SH-SY5Y cells were incubated with indicated concentration of aldioxa, yohimbine, allantoin or aluminium hydrate (Al(OH)3) in the presence of 100 μM clonidine, 5 μM forskolin and 1 mM IBMX for 30 min at 37 °C. Control cells were incubated with 5 μM forskolin and 1 mM IBMX in the presence ( + ) or absence (−) of 100 μM clonidine. Intracellular cAMP levels were determined by ELISA and expressed relative to control (a). Membrane fractions prepared from cells expressing human α-2 adrenergic receptor (b) or human muscarinic M3 receptor (c) were incubated with [3H]-clonidine (20 nM) (b) or [3H]-NMS (2 nM) (c), respectively, in the presence of indicated concentrations of aldioxa, allantoin, aluminium hydrate (Al(OH)3), L-(-)-norepinephrine or yohimbine for 2 h and clonidine- (b) or NMS- (c) binding was determined by the filter-binding assay. Effect of indicated dose of yohimbine on restraint stress (RS)- (d) or clonidine- (e) induced delayed gastric emptying was examined using the phenol red method as described in the legends of Figs 1 and 4. Values are mean ± s.e.m. *P < 0.05; **P < 0.01 (Tukey test). Experiments were replicated at least two times.
To test this idea directly, we examined the binding of aldioxa to the human α-2 adrenergic receptor by carrying out a [3H]-clonidine displacement study on this receptor. As shown in Fig. 6b, as for L-(-)-norepinephrine (an endogenous ligand for the α-2 adrenergic receptor) and yohimbine, aldioxa inhibited clonidine-binding to the human α-2 adrenergic receptor in a dose-dependent manner, suggesting that aldioxa binds to this receptor. We also found that allantoin but not aluminium hydrate inhibited clonidine binding to the α-2 adrenergic receptor (Fig. 6b). On the other hand, aldioxa did not show an inhibitory effect on N-methyl-scopolamine methyl bromide (NMS)-binding to the human muscarinic M3 receptor (Fig. 6c). These results suggest that aldioxa binds specifically to the α-2 adrenergic receptor.
With the above results suggesting that the stimulatory effect of aldioxa on gastric emptying is mediated via its antagonist activity on the α-2 adrenergic receptor, we tested this idea further by examining the effect of yohimbine on delayed gastric emptying. As shown in Fig. 6d, pre-administration of yohimbine suppressed restraint stress-induced delayed gastric emptying. Similar results were observed for clonidine-induced delayed gastric emptying (Fig. 6e).
Effect of aldioxa on gastric compliance in rats
We next examined the effect of aldioxa on gastric compliance measured with a barostat apparatus (see Methods). As shown in Fig. 7a, increasing balloon pressure resulted in an increase in balloon volume. The pre-administration of acotiamide to animals potentiated this increase, as described previously in human18, showing that acotiamide increases gastric compliance. On the other hand, pre-administration of aldioxa did not significantly affect gastric compliance in control rats (Fig. 7a).
Effect of aldioxa on gastric compliance in rats.
Rats fasted for 18 h were orally administered indicated dose of aldioxa (mg kg−1) (a,c), acotiamide (100 mg kg−1) (a,c), allantoin (145 mg kg−1) (d), aluminium hydrate (Al(OH)3) (72 mg kg−1) (d), yohimbine (1 mg kg−1) (e), sucralfate (56 mg kg−1) (e) or vehicle (1% methylcellulose) (a,c–e). Rats were subjected to wrap restraint stress (WRS) for 1 h (b–e). Gastric compliance was measured one hour after the administration of each drug. Values are mean ± s.e.m. *P < 0.05; **P < 0.01 (Tukey test) (versus vehicle (a), versus control (b), versus WRS (c–e). Experiments were replicated at least two times.
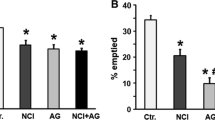
We subsequently examined the effect of aldioxa on gastric compliance in rats subjected to wrap restraint stress. As shown in Fig. 7b, compared to control rats, gastric compliance was decreased in rats subjected to wrap restraint stress. Furthermore, pre-administration of aldioxa or acotiamide increased gastric compliance in these rats (Fig. 7c). These results suggest that administration of aldioxa stimulates gastric compliance under stress conditions.
Finally, we examined the effect of allantoin or aluminium hydrate on gastric compliance. As shown in Fig. 7d, aluminium hydrate but not allantoin increased gastric compliance in rats subjected to wrap restraint stress at a dose equivalent to 200 mg kg−1 of aldioxa. We also found that sucralfate (56 mg kg−1; a dose equivalent to 72 mg kg−1 of aluminium hydrate (or 200 mg kg−1 of aldioxa) in terms of the molecular number of contained aluminium) increased gastric compliance in rats subjected to wrap restraint stress. On the other hand, yohimbine did not affect gastric compliance in rats subjected to wrap restraint stress (Fig. 7e). The results in Fig. 7 suggest that aldioxa achieves its stimulatory action on gastric compliance independently of its inhibitory action against the α-2 adrenergic receptor; the effect on gastric compliance is induced by aldioxa’s aluminium hydrate moiety.
Discussion
A reduced quality of life is a typical consequence of FD in many people suffering from this disease and this in turn has a significant economic impact on the healthcare system. In spite of this, an effective clinical protocol for the treatment of FD has not been established. Although various types of drugs have been developed (such as 5-HT4 agonists and D2 antagonists), most of them have drawbacks such as low efficacy and serious adverse effects8,9. Thus, in order to find new drugs to treat FD, we adopted a strategy of phenotypic screening in animals. Furthermore, to reduce the risk of adverse effects in the clinical setting, we sought to use a drug re-positioning strategy (see introduction).
By screening a library of medicines already used clinically, we found that the oral administration of aldioxa partially suppressed clonidine-induced delayed gastric emptying in mice. Aldioxa was approved in 1971 in Japan and has been used for the prevention and treatment of gastric ulcer and gastritis. We suggest that the stimulatory effect of aldioxa on gastric emptying is independent of its protective effect on the gastric mucosa based on the following: (i) the dose of aldioxa required to affect gastric emptying was much lower than that required to suppress the production of gastric lesions; (ii) other gastroprotective drugs (geranylgeranylacetone and sucralfate) had no effect on delayed gastric emptying.
We found that administration of allantoin but not aluminium hydroxide suppressed the delayed gastric emptying, suggesting that the stimulatory effect of aldioxa on gastric emptying is mediated by its allantoin moiety. This idea is supported by the ineffectiveness on delayed gastric emptying of sucralfate, a drug that also contains aluminium hydroxide.
Aldioxa also suppressed the delayed gastric emptying induced by restraint stress, but did not affect the basal level of gastric emptying in intact mice. Since FD is closely associated with emotional stress, the effectiveness of aldioxa on restraint stress-induced delayed gastric emptying supports it possible beneficial effects on FD patients. On the other hand, since alterations to basal levels of gastric emptying by drugs are related to their adverse effects, the lack of effect of aldioxa on the basal level of gastric emptying in these animals also supports its possible usefulness in FD patients.
It was important to identify the molecular target of aldioxa that mediates its effect on delayed gastric emptying. Since 5-HT4 receptor agonists and D2 antagonists are used clinically to restore gastric emptying in FD patients, we tested the possibility that aldioxa acts as a 5-HT4 receptor agonist or D2 antagonist. However, these possibilities were ruled out by observations that aldioxa restored gastric emptying even in the presence of a 5-HT4 receptor antagonist but did not affect delayed gastric emptying induced by a D2 receptor agonist.
We then tested involvement of the α-2 adrenergic receptor, because aldioxa is effective in overcoming the delayed gastric emptying induced by the α-2 adrenergic receptor agonist clonidine. Treatment of cells with aldioxa or allantoin restored intracellular cAMP levels in the presence of clonidine, with a filter-binding assay revealing that both aldioxa and allantoin inhibit clonidine binding to the human α-2 adrenergic receptor. Furthermore, yohimbine, an α-2 adrenergic receptor antagonist, suppressed restraint stress- or clonidine-induced delayed gastric emptying. All these results support the notion that aldioxa ameliorates delayed gastric emptying through its antagonistic activity on the α-2 adrenergic receptor. Although it is known that α-2 adrenergic receptor antagonism stimulates acetylcholine release from the vague nerve, this receptor has not received much attention as a target of anti-FD drugs32. The results of this study, however, suggest that the α-2 adrenergic receptor could be such a target of drugs to improve gastric emptying in FD patients.
We here show that aldioxa suppresses the delayed gastric emptying induced by restraint stress or administration of clonidine. Since restraint stress-induced delayed gastric emptying was reported to involve an adrenergic pathway7, it is possible that the restoration by aldioxa would work in conditions only involving an adrenergic pathway. Supporting this notion, aldioxa did not affect delayed gastric emptying induced by a D2 receptor agonist. Furthermore, the physiology and anatomy of the rat stomach differ from man33. Thus, it is possible that this drug shows ineffectiveness in FD patients. On the other hand, we found that aldioxa at 200 mg kg−1 (30 times higher than the clinical dose) shows more potent effect on delayed gastric emptying than cisapride at 10 mg kg−1 (60 times higher than the clinical dose), suggesting that the clinical dose of aldioxa shows effectiveness on delayed gastric emptying in human. Therefore, clinical investigations would be required to determine the clinical significance of this effect.
In addition to delayed gastric emptying, impaired gastric accommodation (decrease in gastric compliance) plays an important role in the pathogenesis of FD, particularly PDS34. We therefore examined the effect of aldioxa on gastric compliance and found that, in contrast to acotiamide, orally administered aldioxa did not affect gastric compliance in control rats. We did however find that gastric compliance was decreased in rats subjected to wrap restraint stress and that aldioxa increased gastric compliance under such conditions. Concerning the mechanism governing this effect, we found that, in contrast to its stimulatory effect on gastric emptying, aluminium hydroxide but not allantoin or yohimbine restored gastric compliance decreased by wrap restraint stress. These results suggest that aldioxa increases gastric compliance via a mechanism that is independent of inhibitory effect on the α-2 adrenergic receptor, but dependent on its aluminium hydroxide moiety. Supporting this notion, sucralfate (which contains aluminium hydroxide) increased gastric compliance in rats subjected to wrap restraint stress. However, the mechanism how aluminium hydroxide moiety of aldioxa affects gastric compliance has remained unknown. On the other hand, it also should be noted that this is the first demonstration that acotiamide affects gastric accommodation in animals.
Since the present protocol for treating FD has not resulted in satisfactory outcomes on the whole and a large number of people suffer from this disease, new compounds are currently being developed. However, such an approach involves an enormous financial outlay and the obvious risk of encountering unacceptable side effects during the clinical trial process. Aldioxa might offer a distinct advantage in that its safety has already been clinically demonstrated. Furthermore, because pilot clinical studies are possible for approved medicines, the efficacy (or not) of aldioxa in FD patients can be confirmed without further pre-clinical studies.
In conclusion, the results of this study suggest that aldioxa may be a good candidate drug for treating FD on the basis of its stimulatory effects on both gastric emptying and gastric compliance under conditions where these properties are impaired.
Methods
Chemicals and animals
Methylcellulose, carboxymethylcellulose sodium, ethyl carbamate (urethane), clonidine, apomorphine, yohimbine, aldioxa and phenol red were obtained from Wako Pure Chemical Industries (Osaka, Japan). Cisapride, 3-isobutyl-1-methylxanthine (IBMX), forskolin, fetal bovine serum (FBS), allantoin, L-(-)-norepinephrine, GR113808 and mosapride were purchased from Sigma (St. Louis, MO). Itopride and sucralfate were obtained from LKT laboratories Inc. (St Paul, MN). Saline and Racol® were from Otsuka Pharmaceutical Co. Ltd. (Tokyo, Japan). [13C]-labeled acetic acid was obtained from Cambridge Isotope Laboratories (Andover, MA). Dulbecco’s modified Eagle’s medium and Ham’s F-12 medium were purchased from Nissui Pharmaceutical Co. Ltd. (Tokyo, Japan). An ELISA kit for cAMP was from ENZO Life Sciences (Farmingdale, NY). Aluminium hydroxide was from Nacalai Tesque Inc. (Kyoto, Japan). Membrane fractions prepared from Chinese hamster ovary (CHO)-K1 cells expressing human α-2A adrenergic receptor or human muscarinic M3 receptor, [benzen ring-3H]-clonidine ([3H]-clonidine) (61.3 Ci mmol−1) and [3H]-NMS (85.5 Ci mmol−1) were obtained from Perkin-Elmer Life and Analytical Sciences (Boston, MA). Acotiamide and geranylgeranylacetone were gifted from Zeria Pharmaceutical Co. Ltd. (Saitama, Japan) and Eisai Co. Ltd. (Tokyo, Japan), respectively. ICR mice (8-week-old males, 30–35 g) and Sprague-Dawley rats (12-week-old males, 320–370 g) were obtained from Charles River Laboratories Japan (Yokohama, Japan). Animals were housed under conditions of controlled temperature (22–24 °C) and illumination (12 h light cycle) for 1 or 2 weeks before experiments. The experiments and procedures described here were performed in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health and were approved by the Animal Care Committee of Keio University.
Measurement of gastric emptying using the [13C]-labeled acetic acid breath test
Monitoring of the 13CO2 level in air expired by mice was performed as described previously23 with some modifications. The apparatus was composed of a bottle top filter unit (animal chamber; Steritop filter units, Millipore, Billerica, MA), peristaltic pump (Masterflex, Cole-Palmer Instruments Co., Vernon Hills, IL) and a breath-sampling bag (Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan). The animal chamber was wide enough to allow the mouse to turn around. Mice were fasted for 18 h and had free access to water. Racol containing [13C]-labeled acetic acid (16 mg acetic acid/kg, 5 ml racol/kg) was then intragastrically administered. Expired air containing 13CO2 was collected 5, 10, 15, 20, 25, 30, 40, 50, 60 and 90 min after administration of [13C]-labeled acetic acid. The ventilation volume was 50 ml min−1. The content of 13CO2 in collected expired air was measured with a POC-one analyzer (Otsuka Electronics Co. Ltd., Osaka, Japan). Values are presented as the difference of 13CO2/12CO2 (Δ13CO2 (%)) between each sample and the standard air. For evaluation of the gastric emptying, we determined the half-time for gastric emptying (T1/2).
Measurement of gastric emptying with the phenol red method
Gastric emptying was also monitored by use of the phenol red method as previously described35. Mice were fasted for 18 h and had free access to water, following which 1.5% carboxymethylcellulose sodium salt containing 0.05% phenol red (0.5 ml/mouse) was intragastrically administered. Twenty minutes later, mice were sacrificed. After harvesting the stomach and the gastric content collected and centrifuged at 3,000 rpm for 15 min after treatment with 10 ml of 0.1 M NaHCO3. The amount of phenol red in the supernatant was determined based on the absorbance at 558 nm measured using a microplate reader (Multiscan GO, Thermo Scientific, Yokohama, Japan). Phenol red collected from an animal sacrificed immediately after the described administration procedure was used as the standard sample. Gastric emptying (%) was calculated according to the following equation: (1 − amount of phenol red in test sample/amount of phenol red in standard sample) × 100.
Animal models for delayed gastric emptying and effect of test compounds on this parameter
Three different animal models of delayed gastric emptying were used, with delayed emptying induced by clonidine, apomorphine or restraint stress.
In the clonidine-induced delayed gastric emptying model, 100 μg kg−1 (for phenol red method) or 30 μg kg−1 (for [13C]-labeled acetic acid breath test) clonidine were subcutaneously administered to mice 5 or 15 min before the administration of phenol red or 13C-labeled acetic acid, respectively. In the apomorphine-induced delayed gastric emptying model, mice were subcutaneously administered 5 mg kg−1 apomorphine 30 min before the administration of phenol red.
In the restraint stress-induced delayed gastric emptying model, mice were placed individually into a 50 ml Falcon tube (Becton Dickinson, Franklin Lakes, NJ) for 1 h and then [13C]-labeled acetic acid or phenol red was administered. These tubes were small enough to restrain a mouse so that it is able to breathe but unable to move freely. Control mice were left to move freely in their cages.
In any of these animal models, each test compound was administered intragastrically with 1% methylcellulose (10 ml kg−1) 1 h before the administration of [13C]-labeled acetic acid or phenol red.
Cell culture and determination of the intracellular cAMP level
SH-SY5Y cells were cultured in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 10% FBS, 100 units/ml penicillin and 100 μg ml−1 streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37 °C.
Cells were plated in 24-well plates at a density of 2 × 105 cells/well, incubated for 24 h and then pre-treated with 5 μM forskolin and 1 mM IBMX for 5 min at 37 °C. Cells were then incubated with each test compound in the presence of 100 μM clonidine, 5 μM forskolin and 1 mM IBMX for 30 min at 37 °C. The incubation was stopped by replacing the media with 250 μl ice-cold 0.1N HCl. After 20 min incubation with shaking at room temperature, aliquots were recovered and centrifuged. The amount of cAMP in the supernatant was measured with a cAMP ELISA kit according to the manufacturer’s instructions. Reported values are the means of nine experiments.
Filter-binding assay
The filter-binding assay was performed as described previously36 with some modifications. Membrane fractions prepared from CHO-K1 cells expressing the human α-2 adrenergic receptor or human muscarinic M3 receptor (4 μg protein) were incubated with 20 nM [3H]-clonidine or 2 nM [3H]-NMS, respectively, at room temperature for 1 h in binding buffer (50 mM Tris-HCl (pH 7.4) and 100 mM NaCl) in the presence of each test compound. The sample was passed through a GF/B filter (F7036, Sigma, St. Louis, MO) that was pre-incubated for 30 min with 1.0% polyethylenimine and washed four times with ice-cold PBS (pH 7.6). Filters were then dried for 30 min and the radioactivity remaining on the filter was monitored with a Tri-Carb liquid scintillation counter (PerkinElmer Life and Analytical Sciences, Boston, MA). Non-specific binding to the α-2 adrenergic receptor or muscarinic M3 receptor was determined by examining binding in the presence of yohimbine (10 μM) or atropine (2.5 μM), respectively and specific binding to each receptor was calculated by subtracting the value of non-specific binding. Reported values are the means of three experiments.
Gastric compliance test
Gastric compliance was examined as described previously37 with some modifications. Briefly, rats fasted for 18 h were orally administered each test compound in combination with 1% methylcellulose (2.5 ml/kg). After a 1 h interval (control) or after 1 h of being subjected to wrap restraint stress38, rats were anesthetized with urethane (1.5 g kg−1). A polyethylene bag (maximum volume, 5 ml; maximum diameter, 3 cm) connected to a pair of polyvinyl tubes was introduced into the stomach, with the tubes exiting via the mouth. Air was injected into the balloon to allow placement of the balloon in the stomach. After a 10 min recovery period, the balloon tubes were connected to a barostat (Barostat Distender IIR, Starmedical, Tokyo, Japan) and the pressure inside the balloon was increased stepwise at 1 min intervals. The balloon volume increased gradually until it reached a plateau. The difference in balloon volume between the initial and the final (plateau) levels was defined as gastric compliance.
Gastric damage assay
The gastric ulcerogenic response was examined as described previously39,40 with some modifications. Mice fasted for 18 h were orally pre-administered each test compound and after 1 h, orally administered indomethacin (20 mg kg−1). After 8 h, the animals were sacrificed, their stomachs were removed and the gastric mucosal lesion area was measured by an observer unaware of the treatment the animals had received. Calculation of the scores involved measuring the area of all the lesions (expressed in square millimetres) and summing the values to give an overall lesion index.
Statistical analysis
Each value is expressed as the mean ± S.E.M. Two-way ANOVA followed by the Tukey test or the Student’s t-test for unpaired results was used to evaluate differences between more than two groups or between two groups, respectively. Differences were considered to be significant for values of P < 0.05.
Additional Information
How to cite this article: Asano, T. et al. Aldioxa improves delayed gastric emptying and impaired gastric compliance, pathophysiologic mechanisms of functional dyspepsia. Sci. Rep. 5, 17519; doi: 10.1038/srep17519 (2015).
References
El-Serag, H. B. & Talley, N. J. Systemic review: the prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther 19, 643–654 (2004).
Lacy, B. E. et al. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther 38, 170–177 (2013).
Choung, R. S. et al. Do distinct dyspepsia subgroups exist in the community? A population-based study. Am J Gastroenterol 102, 1983–1989 (2007).
Bisschops, R. et al. Relationship between symptoms and ingestion of a meal in functional dyspepsia. Gut 57, 1495–1503 (2008).
Timmons, S., Liston, R. & Moriarty, K. J. Functional dyspepsia: motor abnormalities, sensory dysfunction and therapeutic options. Am J Gastroenterol 99, 739–749 (2004).
Ogiso, K., Asakawa, A., Amitani, H. & Inui, A. Ghrelin: a gut hormonal basis of motility regulation and functional dyspepsia. J Gastroenterol Hepatol 26 Suppl 3, 67–72 (2011).
Nakade, Y. et al. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am J Physiol Regul Integr Comp Physiol 288, R427–432 (2005).
Brun, R. & Kuo, B. Functional dyspepsia. Therap Adv Gastroenterol 3, 145–164 (2010).
Holtmann, G. & Gapasin, J. Failed therapy and directions for the future in dyspepsia. Dig Dis 26, 218–224 (2008).
Bran, S., Murray, W. A., Hirsch, I. B. & Palmer, J. P. Long QT syndrome during high-dose cisapride. Arch Intern Med 155, 765–768 (1995).
Haug, T. T. et al. Low vagal activity as mediating mechanism for the relationship between personality factors and gastric symptoms in functional dyspepsia. Psychosom Med 56, 181–186 (1994).
Hausken, T. et al. Low vagal tone and antral dysmotility in patients with functional dyspepsia. Psychosom Med 55, 12–22 (1993).
Suzuki, H. & Hibi, T. Acotiamide (Z-338) as a possible candidate for the treatment of functional dyspepsia. Neurogastroenterol Motil 22, 595–599 (2010).
Yokotani, K., Okuma, Y., Nakamura, K. & Osumi, Y. Release of endogenous acetylcholine from a vascularly perfused rat stomach in vitro; inhibition by M3 muscarinic autoreceptors and alpha-2 adrenoceptors. J Pharmacol Exp Ther 266, 1190–1195 (1993).
Kusunoki, M., Taniyama, K. & Tanaka, C. Dopamine regulation of [3H]acetylcholine release from guinea-pig stomach. J Pharmacol Exp Ther 234, 713–719 (1985).
Janssen, P. et al. Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther 33, 880–894 (2011).
Tack, J. et al. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 115, 1346–1352 (1998).
Kusunoki, H. et al. Therapeutic efficacy of acotiamide in patients with functional dyspepsia based on enhanced postprandial gastric accommodation and emptying: randomized controlled study evaluation by real-time ultrasonography. Neurogastroenterol Motil 24, 540–545, e250–541 (2012).
Mizushima, T. Drug discovery and development focusing on existing medicines: drug re-profiling strategy. J Biochem 149, 499–505 (2011).
Fukawa, K. et al. [Experimental studies on gastric ulcer (5). Endoscopical evaluation of healing processes of acetic acid ulcer in rats (1). Ulcer reducing process]. Nihon Yakurigaku Zasshi 81, 167–174 (1983).
Ohbayashi, S. et al. [The effect of aldioxa on the formation of gastritis induced with sodium hydroxide]. Nihon Yakurigaku Zasshi 96, 255–263 (1990).
Tanaka, T. et al. Effects of EM574 and cisapride on gastric contractile and emptying activity in normal and drug-induced gastroparesis in dogs. J Pharmacol Exp Ther 287, 712–719 (1998).
Matsumoto, K. et al. Validation of 13C-acetic acid breath test by measuring effects of loperamide, morphine, mosapride and itopride on gastric emptying in mice. Biol Pharm Bull 31, 1917–1922 (2008).
Cahen, R. & Pessonnier, A. [Pharmacological study of dihydroxyaluminum allantoinate and chlorhydroxyaluminum allantoinate. III. Anti-ulcerous effect]. Ann Pharm Fr 20, 704–713 (1962).
Cahen, R. & Pessonnier, A. [Pharmacological study of dihydroxyaluminum allantoinate and chlorhydroxyaluminum allantoinate. IV. Effect on experimental drug-induced ulcer]. Ann Pharm Fr 21, 215–222 (1963).
Lee, M. Y. et al. Protective effects of allantoin against ovalbumin (OVA)-induced lung inflammation in a murine model of asthma. Int Immunopharmacol 10, 474–480 (2010).
Konturek, S. J. et al. Intragastric pH in the gastroprotective and ulcer-healing activity of aluminum-containing antacids. Digestion 49, 140–150 (1991).
Seto, K. et al. Acotiamide hydrochloride (Z-338), a novel prokinetic agent, restores delayed gastric emptying and feeding inhibition induced by restraint stress in rats. Neurogastroenterol Motil 20, 1051–1059 (2008).
Lee, T. H. et al. Tetrahydroberberine, an isoquinoline alkaloid isolated from corydalis tuber, enhances gastrointestinal motor function. J Pharmacol Exp Ther 338, 917–924 (2011).
Gale, J. D. et al. GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br J Pharmacol 111, 332–338 (1994).
Limon-Boulez, I. et al. Partial agonist clonidine mediates alpha(2)-AR subtypes specific regulation of cAMP accumulation in adenylyl cyclase II transfected DDT1-MF2 cells. Mol Pharmacol 59, 331–338 (2001).
Tack, J., Caenepeel, P., Corsetti, M. & Janssens, J. Role of tension receptors in dyspeptic patients with hypersensitivity to gastric distention. Gastroenterology 127, 1058–1066 (2004).
Kararli, T. T. Comparison of the gastrointestinal anatomy, physiology and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 16, 351–380 (1995).
Kindt, S. & Tack, J. Impaired gastric accommodation and its role in dyspepsia. Gut 55, 1685–1691 (2006).
Kawachi, M. et al. Acotiamide hydrochloride (Z-338) enhances gastric motility and emptying by inhibiting acetylcholinesterase activity in rats. Eur J Pharmacol 666, 218–225 (2011).
Asano, T., Makise, M., Takehara, M. & Mizushima, T. Interaction between ORC and Cdt1p of Saccharomyces cerevisiae. FEMS Yeast Res 7, 1256–1262 (2007).
Uchida, M. & Shimizu, K. Evaluation of adaptive relaxation of the rat stomach using an orally inserted balloon instead of surgical intervention by demonstrating the effects of capsaicin and Nomega-nitro-L-arginine methylester. J Smooth Muscle Res 48, 97–104 (2012).
Ait-Belgnaoui, A. et al. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut 55, 1090–1094 (2006).
Tomisato, W. et al. Role of direct cytotoxic effects of NSAIDs in the induction of gastric lesions. Biochem Pharmacol 67, 575–585 (2004).
Takeuchi, K., Ueki, S. & Okabe, S. Importance of gastric motility in the pathogenesis of indomethacin-induced gastric lesions in rats. Dig Dis Sci 31, 1114–1122 (1986).
Acknowledgements
We thank to Dr. Masayuki Uchida (Food Science Institute, Meiji Co. Ltd.) for technical advice for gastric compliance analysis. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Health, Labour and Welfare of Japan, as well as Center of Innovation Program from Japan Science and Technology Agency, Scientific Technique Research Promotion Program for Agriculture, Forestry and Food Industry and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Contributions
Conception and design: T.A. and T.M.; Analysis and interpretation: T.A., S.A., S.S., K.T. and K.-I.T.; Drafting the manuscript for important intellectual content: T.A. and T.M.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Asano, T., Aida, S., Suemasu, S. et al. Aldioxa improves delayed gastric emptying and impaired gastric compliance, pathophysiologic mechanisms of functional dyspepsia. Sci Rep 5, 17519 (2015). https://doi.org/10.1038/srep17519
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17519
- Springer Nature Limited