Abstract
Background/Aims
We examined the contributions of gastric emptying and duodenogastric bile reflux in the formation of gastric antral ulcers induced by NSAIDs in mice.
Methods
We used the murine re-fed indomethacin (IND) experimental ulcer model. Outcome measures included the appearance of gastric lesions 24 h after IND treatment and the assessment of gastric contents and the concentration of bile acids 1.5 h after re-feeding. The effects of atropine, dopamine, SR57227 (5-HT3 receptor agonist), apomorphine, ondansetron, haloperidol, and dietary taurocholate and cholestyramine were also examined.
Results
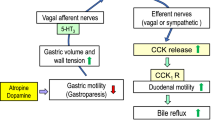
IND (10 mg/kg, s.c.) induced severe lesions only in the gastric antrum in the re-fed model. The antral lesion index and the amount of food intake during the 2-h refeeding period were positively correlated. Atropine and dopamine delayed gastric emptying, increased bile reflux, and worsened IND-induced antral lesions. SR57227 and apomorphine worsened antral lesions with increased bile reflux. These effects were prevented by the anti-emetic drugs ondansetron and haloperidol, respectively. The anti-emetic drugs markedly decreased the severity of antral lesions and the increase of bile reflux induced by atropine or dopamine without affecting delayed gastric emptying. Antral lesions induced by IND were increased by dietary taurocholate but decreased by the addition of the bile acid sequestrant cholestyramine.
Conclusions
These results suggest that gastroparesis induced by atropine or dopamine worsens NSAID-induced gastric antral ulcers by increasing duodenogastric bile reflux via activation of 5-HT3 and dopamine D2 receptors.










Similar content being viewed by others
References
Taylor RT, Huskisson EC, Whitehouse GH, Hart FD, Trapnell DH. Gastric ulceration occurring during indomethacin therapy. Br Med J 1968;4:734–737.
Roth SH, Bennett RE. Nonsteroidal anti-inflammatory drug gastropathy. Recognition and response. . Arch Intern Med 1987;147:2093–2100.
Talley NJ, Locke GR 3rd, Lahr BD et al. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut 2006;55:933–939.
McCarthy DM. Nonsteroidal antiinflammatory drug-induced ulcers: management by traditional therapies. Gastroenterology 1989;96:662–674.
Satoh H, Urushidani T. Soluble dietary fiber can protect the gastrointestinal mucosa against nonsteroidal anti-inflammatory drugs in mice. Dig Dis Sci 2016;61:1903–1914.
Agrawal NM, Campbell DR, Safdi MA, Lukasik NL, Huang B, Haber MM. Superiority of lansoprazole vs ranitidine in healing nonsteroidal anti-inflammatory drug-associated gastric ulcers: results of a double-blind, randomized, multicenter study. NSAID-associated gastric ulcer study group. Arch Intern Med 2000;160:1455–1461.
Campbell DR, Haber MM, Sheldon E et al. Effect of H. pylori status on gastric ulcer healing in patients continuing nonsteroidal anti-inflammatory therapy and receiving treatment with lansoprazole or ranitidine. Am J Gastroenterol 2002;97:2208–2214.
Yeomans ND, Svedberg LE, Naesdal J. Is ranitidine therapy sufficient for healing peptic ulcers associated with non-steroidal anti-inflammatory drug use? Int J Clin Pract 2006;60:1401–1407.
Satoh H, Akiba Y, Urushidani T. Proton pump inhibitors prevent gastric antral ulcers induced by NSAIDs via activation of capsaicin-sensitive afferent nerves in mice. Dig Dis Sci 2020;65:2580–2594.
Takeuchi K, Ueki S, Okabe S. Importance of gastric motility in the pathogenesis of indomethacin-induced gastric lesions in rats. Dig Dis Sci 1986;31:1114–1122.
Ueki S, Takeuchi K, Okabe S. Gastric motility is an important factor in the pathogenesis of indomethacin-induced gastric mucosal lesions in rats. Dig Dis Sci 1988;33:209–216.
Müller-Lissner SA, Fimmel CJ, Sonnenberg A et al. Novel approach to quantify duodenogastric reflux in healthy volunteers and in patients with type I gastric ulcer. Gut 1983;24:510–518.
Sonnenberg A, Müller-Lissner SA, Schattenmann G, Siewert JR, Blum AL. Duodenogastric reflux in the dog. Am J Physiol 1982;242:G603–G607.
Davenport HW. Destruction of the gastric mucosal barrier by detergents and urea. Gastroenterology 1968;54:175–181.
Duane WC, Wiegand DM. Mechanism by which bile salt disrupts the gastric mucosal barrier in the dog. J Clin Invest 1980;66:1044–1049.
Armstrong D, Rytina ER, Murphy GM, Dowling RH. Gastric mucosal toxicity of duodenal juice constituents in the rat. Acute studies using ex vivo rat gastric chamber model. Dig Dis Sci 1994;39:327–339.
Nagahata Y, Azumi Y, Kawakita N, Wada T, Saitoh Y. Inhibitory effect of dopamine on gastric motility in rats. Scand J Gastroenterol 1995;30:880–885.
Levein NG, Thörn SE, Wattwil M. Dopamine delays gastric emptying and prolongs orocaecal transit time in volunteers. Eur J Anaesthesiol 1999;16:246–250.
Kindt S, Tack J. Impaired gastric accommodation and its role in dyspepsia. Gut 2006;55:1685–1691.
Tack J, Camilleri M. New developments in the treatment of gastroparesis and functional dyspepsia. Curr Opin Pharmacol 2018;43:111–117.
Feinle C, Read NW. Ondansetron reduces nausea induced by gastroduodenal stimulation without changing gastric motility. Am J Physiol 1996;271:G591–G597.
Sanger GJ, Andrews PLR. A history of drug discovery for treatment of nausea and vomiting and the implications for future research. Front Pharmacol 2018;9:913.
Parthasarathy G, Ravi K, Camilleri M et al. Effect of neostigmine on gastroduodenal motility in patients with suspected gastrointestinal motility disorders. Neurogastroenterol Motil 2015;27:1736–1746.
Uchida M, Yamato S, Shimizu K, Amano T, Ariga H. Dual role of mosapride citrate hydrate on the gastric emptying evaluated by the breath test in conscious rats. J Pharmacol Sci 2013;121:282–287.
Seto Y, Yoshida N, Kaneko H. Effects of mosapride citrate, a 5-HT4-receptor agonist, on gastric distension-induced visceromotor response in conscious rats. J Pharmacol Sci 2011;116:47–53.
Mashige F, Tanaka N, Maki A, Kamei S, Yamanaka M. Direct spectrophotometry of total bile acids in serum. Clin Chem 1981;27:1352–1356.
Bachy A, Héaulme M, Giudice A et al. SR 57227A: a potent and selective agonist at central and peripheral 5-HT3 receptors in vitro and in vivo. Eur J Pharmacol 1993;237:299–309.
Depoortère R, Barret-Grévoz C, Bardin L, Newman-Tancredi A. Apomorphine-induced emesis in dogs: differential sensitivity to established and novel dopamine D2/5-HT1A antipsychotic compounds. Eur J Pharmacol 2008;597:34–38.
Markham A, Sorkin EM. An update of its therapeutic use in chemotherapy-induced and postoperative nausea and vomiting. Drugs 1993;45:931–952.
Schwalbe T, Kaindl J, Hübner H, Gmeiner P. Potent haloperidol derivatives covalently binding to the dopamine D2 receptor. Bioorg Med Chem 2017;25:5084–5094.
Acosta A, Camilleri M. Prokinetics in gastroparesis. Gastroenterol Clin North Am 2015;44:97–111.
Gotthard R, Bodemar G, Tjädermo M, Tobiasson P, Walan A. High gastric bile acid concentration in prepyloric ulcer patients. Scand J Gastroenterol 1985;20:439–446.
Rydning A, Berstad A. Intragastric bile acid concentrations in healthy subjects and in patients with gastric and duodenal ulcer and the influence of fiber-enriched wheat bran in patients with gastric ulcer. Scand J Gastroenterol 1985;20:801–804.
Duplessis DJ. Pathogenesis of gastric ulceration. Lancet 1965;1:974–978.
Rhodes J, Barnardo DE, Phillips SF, Rovelstad RA, Hofmann AF. Increased reflux of bile into the stomach in patients with gastric ulcer. Gastroenterology 1969;57:241–252.
Yokoyama M, Tatsuta M, Baba M, Kitamura Y. Bile reflux: a possible cause of stomach ulcer in nontreated mutant mice of W/Wv genotype. Gastroenterology 1982;82:857–863.
Azuma T, Dojyo M, Ito S et al. Bile reflux due to disturbed gastric movement is a cause of spontaneous gastric ulcer in W/Wv mice. Dig Dis Sci 1999;44:1177–1183.
Silen W, Forte JG. Effects of bile salts on amphibian gastric mucosa. Am J Physiol 1975;228:637–644.
Schindlbeck NE, Heinrich C, Stellaard F, Paumgartner G, Müller-Lissner SA. Healthy controls have as much bile reflux as gastric ulcer patients. Gut 1987;28:1577–1583.
Li D, Zhang J, Yao WZ et al. The relationship between gastric cancer, its precancerous lesions and bile reflux: a retrospective study. J Dig Dis 2020;21:222–229.
Režen T, Rozman D, Kovács T et al. The role of bile acids in carcinogenesis. Cell Mol Life Sci 2022;79:243.
Noto JM, Piazuelo MB, Shah SC et al. Iron deficiency linked to altered bile acid metabolism promotes Helicobacter pylori-induced inflammation-driven gastric carcinogenesis. J Clin Invest. 2022. https://doi.org/10.1172/JCI147822.
Lang IM. Physiology of the digestive tract correlates of vomiting. J Neurogastroenterol Motil. 2023;29:20–30.
Mitchelson F. Pharmacological agents affecting emesis. A review (Part I). Drugs 1992;43:295–315.
Belkacemi L, Darmani NA. Dopamine receptors in emesis: molecular mechanisms and potential therapeutic function. Pharmacol Res 2020;161:105–124.
Currò D, Ipavec V, Preziosi P. Neurotransmitters of the non-adrenergic non-cholinergic relaxation of proximal stomach. Eur Rev Med Pharmacol Sci 2008;12:53–62.
Glatzle J, Sternini C, Robin C et al. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology 2002;123:217–226.
Minami M, Endo T, Hirafuji M et al. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther 2003;99:149–165.
Takeuchi K, Miyazawa T, Tanaka A, Kato S, Kunikata T. Pathogenic importance of intestinal hypermotility in NSAID-induced small intestinal damage in rats. Digestion 2002;66:30–41.
Akiba Y, Maruta K, Narimatsu K et al. FFA2 activation combined with ulcerogenic COX inhibition induces duodenal mucosal injury via the 5-HT pathway in rats. Am J Physiol Gastrointest Liver Physiol 2017;313:G117–G128.
Acknowledgments
The authors are greatly indebted to Ms. Ai Sato, Ms. Chihiro Takagi, Ms. Miki Horikawa and Ms. Fumika Kotera, students in our Department, Doshisha Women’s College of Liberal Arts, for their technical assistance, and Drs. Y. Amagase and Y. Mizukawa for valuable discussions and suggestions.
Funding
Doshisha Women’s College of Liberal Arts (TU), VA Merit Review I01BX001245 (JDK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethical approval
Experimental protocols were approved by the Animal Research Committees at Doshisha Women’s College of Liberal Arts, Kodo, Kyotanabe, Kyoto, Japan (Approval Number Y16-031).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Satoh, H., Akiba, Y., Urushidani, T. et al. Gastroparesis Worsens Indomethacin-Induced Gastric Antral Ulcers by Bile Reflux via Activation of 5-HT3 and Dopamine D2 Receptors in Mice. Dig Dis Sci 68, 3886–3901 (2023). https://doi.org/10.1007/s10620-023-08086-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08086-x