Abstract
There is a relative scarcity of large-scale population studies investigating the relationship between the insulin resistance index of homeostasis model assessment (HOMA-IR) and vascular damage. Therefore, we assessed the association between HOMA-IR and vascular damage in adults aged 18 years and older in China. A total of 17,985 research subjects were included. Vascular damage markers and relevant laboratory tests were measured. HOMA-IR was calculated as (fasting insulin * fasting blood glucose)/22.5. Vascular damage included arteriosclerosis (ba-PWV > 1800 cm/s), peripheral artery disease (ABI < 0.9), and microalbuminuria (UACR > 30 mg/g). The relationship between HOMA-IR and vascular damage was analyzed using the RCS. The restricted cubic spline (RCS) analysis suggested that HOMA-IR was nonlinearly associated with arteriosclerosis (P for no-liner < 0.01), peripheral artery disease (P for no-liner < 0.01), and microalbuminuria (P for no-liner < 0.01). Further segmented regression analyses revealed that in study subjects with HOMA-IR < 5, we found that HOMA-IR was associated with an increased OR for arteriosclerosis (OR: 1.36, 95% CI (1.28, 1.45), P < 0.01), peripheral artery disease (OR: 1.33, 95% CI (1.10, 1.60), P < 0.01) and microalbuminuria (OR: 1.59, 95% CI (1.49, 1.70), P < 0.01). HOMA-IR is an independent risk factor for vascular damage, both macrovascular and microvascular. The phenomenon of saturation of HOMA-IR with vascular damage needs further investigation.
Similar content being viewed by others
Introduction
Insulin resistance (IR) is a medical condition characterized by a significant reduction in the biological impact of the body's tissues or organs on the appropriate level of insulin, the body's reactive production of substantial amounts of insulin1,2, and disorders of glucose and lipid metabolism resulting from severe hyperinsulinemia and reduced sensitivity of glucose to the insulin response, which are common causes of numerous diseases3,4,5,6,7.
Dysfunction of endothelial cells caused by IR is the reason for vascular damage. There are two aspects of vascular damage: microvascular lesions and macrovascular lesions. The brachial-ankle pulse wave velocity (baPWV) responds to medium to large arterial stiffness8, and the baPWV is independently associated with adverse cardiovascular events in Asian populations9,10. An ankle-brachial index (ABI) < 0.9 has been recognized as a reliable marker of peripheral arterial occlusion, and patients with an ABI < 0.9 are at a 3–4 times greater risk of cardiovascular death11,12. The urinary albumin-to-creatinine ratio (UACR), which is commonly used to assess renal function, is primarily responsive to microvascular disease. Microalbuminuria (MAU) is a marker of systemic endothelial damage and can predict the development of microvascular events as well as macrovascular events13. The detection of MAU can indicate systemic vascular disease and has a predictive effect on coronary heart disease, diabetic vascular complications, and hypertensive vascular disease14.
The hyperinsulinemic-euglycemic clamp technique (HECT) is currently the gold standard for treating IR, which is limited in its application due to the complexity of the technique and poor subject compliance. The insulin resistance index of homeostasis model assessment (HOMA-IR), which uses insulin and glucose levels derived from the fasting state and correlates well with the HECT, is more suitable for practical application.
A correlation between vascular damage and IR has been reported in previous studies15,16,17. However, most of the related studies have focused on the relationships between different indices calculated based on abdominal obesity, blood lipid composition and fasting glucose and vascular damage. Therefore, this study explored the relationship between the insulin resistance index (HOMA-IR) and vascular damage.
Materials and methods
Study population
Patients who were continuously selected for physical examination in the Department of Health Medicine of the second Medical Center of the PLA General Hospital from January 2018 to December 2019. Participants with age < 18 years old, heart failure, renal failure, acute or chronic inflammatory disease, tumor, pregnancy, or inability to provide informed consent were excluded. Finally, A total of 17,985 subjects were included in the study. All the subjects signed an informed consent form. The study protocol was approved by the Medical Ethics Committee of the General Hospital of the Chinese People's Liberation Army (PLA). Throughout the research process, the researchers adhered strictly to relevant regulations.
Covariate data
All the individuals underwent clinical examinations and anthropometric measurements by experienced medical teams. Height (cm) was measured without shoes on (within 0.1 cm), and Weight (kg) was measured without a heavy coat (within 0.1 kg). Blood pressure (mmHg) measurements were taken with an automatic electronic sphygmomanometer after the participants had rested for 5–10 min. The collection of smoking and drinking status was completed through a brief questionnaire. The smoking status of the subjects was determined by asking, "Do you currently smoke, smoke every day, not smoke every day, or not smoke at all?" The drinking status of participants was determined by asking "Do you currently drink alcohol, how often do you drink, or do you not drink at all?" History of diagnosed diseases and medication use were obtained from hospital medical records and confirmed by a medical record review.
Biochemical parameters
Venous blood was collected from all subjects following an overnight fast according to the quality control and testing standards of the Clinical Laboratory of PLA General Hospital. Fasting insulin levels were measured with the Elecsys insulin radioimmunoassay kit (Elecsys Diagnostics, Roche, Swiss). Hemoglobin A1c (HbA1c) was measured using high performance liquid chromatography (HLC-723@G8, TOSOH, Japan). Fasting glucose, lipids profile, creatinine, and uric acid were detected by automatic biochemical analyzer (Cobas c 702, Roche, Swiss). Experimental methods and procedures are standardized laboratory protocols and guidelines. Midstream urine was collected in the morning, and urine albumin and urine creatinine concentrations were measured by chemiluminescent immunoassay. UACR was calculated by dividing urine albumin (mg) by urine creatinine (g). The HOMA-IR was calculated as (fasting insulin [μIU/mL] × fasting glucose [mmol/L])/22.518.
Measurement of baPWV and ABI
Arterial stiffness was assessed by the baPWV, which was measured using an automated PWV/ABI analyzer (VP-1000; Colin Co., Ltd., Komaki, Japan), and the ABI was measured simultaneously. Brachial and posttibial pressure waveforms were detected by cuffs wrapped around all four extremities connected to a plethysmographic sensor and an oscillometric sensor. Accordingly, the transit time between the brachial and posttibial pressure waveforms (ΔTba) was determined, the distance traveled from the brachium to the ankle (La − Lb) was automatically calculated based on the participant's height, and the baPWV was calculated using the formula (La − Lb)/ΔTba. The ABI was calculated as the ratio of the ankle systolic pressure divided by the brachial artery systolic pressure.
Vascular damage was assessed using the baPWV and ABI, which were measured using an automated PWV/ABI analyzer (VP-1000; Colin Co., Ltd., Komaki, Japan). The pressure waveforms of the brachial and posterior tibial arteries were detected using cuffs wrapped around the limbs, which were connected to volume plethysmographic sensors and oscillometric sensors. Therefore, the time delay between the pressure waveforms of the brachial and tibial arteries (ΔTba) was determined, and the traveling distance from the brachial to the ankle (La − Lb) was automatically calculated based on the participant's height. The baPWV was then calculated using the formula (La − Lb)/ΔTba. The ABI was recorded as the ratio of ankle systolic pressure to brachial systolic pressure.
Statistical methods
Continuous variables are reported as means ± standard deviations, and categorical variables are expressed as percentages of the composition ratio. Analysis of variance (ANOVA) and chi-square tests were used to compare the demographic and clinical characteristics of the different HOMA-IR quartile groups. Possible nonlinear relationships between the change in HOMA-IR and vascular damage were examined by a logistic regression model with restricted cubic spline (RCS). We conducted RCS with 4 knots at the 5th, 35th, 65th, and 90th centiles to flexibly model the association. RCS curves were plotted to analyze the relationships between HOMA-IR and arteriosclerosis, lower limb vascular closure and proteinuria and to determine the inflection point of HOMA-IR. The data were divided into two distinct segments based on this inflection point. When interpreting the results of an RCS analysis, the median value of the predictor variable was chosen as the reference value. Odd ratio (OR) and 95% confidence intervals (95%CI) were calculated using univariate and multivariate logistic regression models. Model 1 was unadjusted for the relevant variables, Model 2 was adjusted for age and sex, and Model 3 was adjusted for Model 2 + low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), triglyceride (TG), uric acid (UA), creatinine (Cr), body mass index (BMI), blood pressure, diabetes mellitus (DM), coronary heart disease(CHD), and cerebrovascular disease(CeVD). Statistical analysis was performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA) and R software, version 4.2.2, along with MSTATA software (www.mstata.com), and P < 0.05 was considered to indicate statistical significance.
Disorders of vascular damage
In this study, vascular damage included arteriosclerosis (baPWV > 1800 cm/s)19, peripheral artery disease (ABI < 0.9)11, and microalbuminuria (UACR > 30 mg/g)20.
Ethical approval
The study was carried out in accordance with the Helsinki Declaration and Chinese Law. All participants gave informed consent before participation in the study. The ethical review of this study was conducted by the Chinese PLA General Hospital.
Informed consent
All participants provided written informed consent.
Results
Clinical characteristics of the study population
A total of 17,985 subjects were included in the study. Of these, 12,146 were men and 5839 were women. Compared to women, men who appeared to have higher blood pressure, BMI, uric acid, creatinine, and triglycerides were more likely to have a poor lifestyle and were more likely to suffer from arteriosclerosis, lower extremity arterial occlusion, and microalbuminuria. Similarly, the prevalence of hypertension, diabetes, and coronary heart disease is greater in men than in women (Table 1).
RCS Analysis of HOMA-IR and Vascular Injury
We used restricted cubic spline flexibility to model and visualize the relationship between HOMA-IR and vascular damage. The RCS analysis suggested a nonlinear association of HOMA-IR with arteriosclerosis (P for no-liner < 0.01), peripheral artery disease (p for no-liner < 0.01), and microalbuminuria (P for no-liner < 0.01). The inflection point of the RCS curve was identified at HOMA-IR = 5, representing a turning point in the relationship between HOMA-IR and vascular damage (Fig. 1).
Further segmented regression analyses revealed that in study subjects with HOMA-IR < 5, we found that HOMA-IR was associated with an increased OR for arteriosclerosis (OR: 1.36, 95% CI (1.28, 1.45), P < 0.01), peripheral artery disease (OR: 1.33, 95% CI (1.10, 1.60), P < 0.01) and microalbuminuria (OR: 1.59, 95% CI (1.49, 1.70), P < 0.01). These relationships were stable after adjusting for LDL, HDL, TG, TC, BMI, blood pressure, hypertension, diabetes mellitus, coronary heart disease, smoking history, and alcohol consumption history. Among the study participants with a HOMA-IR ≥ 5, univariate logistic regression revealed an increased risk of arteriosclerosis (OR: 1.04, 95% CI (1.01, 1.06), P < 0.01) and microalbuminuria with increasing HOMA-IR, while the presence of peripheral artery disease was not statistically significant. No significant differences were found for any of the three indicators of vascular damage after adjusting for relevant influencing factors (Table 2).
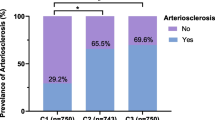
Subjects in the HOMA-IR ≥ 5 subgroup were further grouped according to HOMA-IR quartiles. The means (95% CIs) of the age-adjusted baPWV ABI and UACR of the different HOMA-IR groups (Quartiles 1–4) were (1483.09 (1461.63, 1504.54) vs. 1478.48 (1456.52, 1500.43) vs. 1497.88 (1476.92, 1518.84) vs. 1529.22 (1506.31, 1552.13)cm/s, P < 0.05), (1.09 (1.08, 1.10) vs. 1.09 (1.08, 1.10) vs. 1.08 (1.07, 1.10) vs. 1.08 (1.07, 1.09), P > 0.05) and (23.38 (14.76, 32.00) vs. 24.10 (15.23, 32.96) vs. 47.97 (23.83, 72.12) vs. 83.20 (44.50, 121.88)mg/g, P < 0.05), respectively (Fig. 2). Additionally, the incidence of arteriosclerosis, peripheral artery disease, and microalbuminuria increased with increasing quartiles (Fig. 3).
Subgroup analyses for the association between HOMA-IR and vascular damage
Among the study subjects with a HOMA-IR < 5, all subgroups had consistent HOMA-IR values. Interaction analysis revealed that the correlation between HOMA-IR and arteriosclerosis was stronger in people under 50 years old (P for interaction = 0.01). Among the study subjects with HOMA-IR ≥ 5, the subgroups remained consistent overall, and there was no interaction among the groups (Table 3).
Discussion
In this study, we found that insulin resistance was independently associated with vascular damage and that the incidence of vascular damage did not increase linearly with increasing HOMA-IR. RCS analysis revealed a nonlinear correlation between vascular senescence and insulin resistance.
The correlation between IR and vascular damage has been described in previous studies. IR is associated with hyperinsulinemia, abnormalities in glucolipid metabolism, and an inflammatory state, all of which are risk factors for arteriosclerosis. A study in a nondiabetic population in the United States showed a positive association between HOMA-IR and arteriosclerosis. A positive association between HOMA-IR and TyG, another surrogate for insulin resistance, has also been reported with arteriosclerosis in China19,21,22,23, and similar findings have been reported in South Korea, another Asian country16,24. Similarly, a parallel phenomenon was found in our study.
MAU is commonly used to assess the renal functional status of people with diabetes, while MAU has been found to be closely associated with cardiac microangiopathy25,26, which is a risk factor for coronary artery disease (CAD) or heart failure25,27,28. Studies have shown that insulin resistance can trigger podocyte damage29,30, activation of the RASS system31, and chronic inflammation32, leading to MAU. The results showed a positive correlation between HOMA-IR and UACR, suggesting that HOMA-IR is a risk factor for UACR. This finding is consistent with those of previous studies. In Japanese patients with type 2 diabetes mellitus who had developed MAU, MAU was significantly associated with insulin resistance, and IR was significantly associated with microalbuminuria in a study of insulin resistance and arteriosclerosis in 982 nondiabetic patients by Mykkänen et al.33.
We found a nonlinear relationship between HOMA-IR and vascular damage with saturation. We have noted some reports that may support this finding. The metabolic score for insulin resistance (METS-IR) showed a nonlinear relationship with diabetes onset, with a saturation effect, and researchers have attributed this phenomenon to differences in subject selection and covariates34. A study has shown a nonlinear correlation between insulin resistance and subclinical myocardial injury, with insulin resistance being a protective factor below the threshold and a risk factor above the threshold35. The association between insulin resistance and subclinical myocardial injury was found to be statistically significant only in participants without diabetes35. There was a significant dose‒response relationship between the TyG index and the risk of arterial stiffness (P nonlinear = 0.005)36. Uncovering the pathophysiologic basis of the nonlinear relationship between insulin resistance and associated adverse outcomes may contribute to the prevention and treatment of chronic diseases associated with insulin resistance.
We found that there is an inflection point in the relationship between vascular damage and HOMA-IR. When HOMA ≥ 5, the risk of vascular damage tends to stabilize as HOMA-IR increases. This does not mean that the risk of vascular damage caused by insulin resistance has disappeared, but that it has reached saturation. In other words, when HOMA-IR ≥ 5, a smaller improvement in insulin resistance will have less benefit. Xie et al. found that there is a nonlinear correlation between MetS-IR and the risk of prediabetes, with an obvious saturation effect point, and when MetS-IR is greater than the value of the saturation effect point, the risk of prediabetes gradually tends to stabilize37. A study on the relationship between MetS-IR and NAFLD in non-obese populations found a clear saturation point between MetS-IR and NAFLD38. This underscores the importance of early assessment and intervention against insulin resistance.
However, this study has several limitations. Due to the cross-sectional design of the present study, we were unable to determine a causal relationship between HOMA-IR and vascular damage. The participants were from medical examination centers in China, which may have limited the generalizability of the findings. Finally, we were unable to adjust for several potential confounding factors that may have influenced the results, including medication history and physical activity.
Conclusion
HOMA-IR is an independent risk factor for vascular damage, both macrovascular and microvascular. The phenomenon of saturation of HOMA-IR with vascular damage needs further investigation.
Data availability
The datasets used in the study are available from the corresponding author upon reasonable request.
Abbreviations
- HOMA-IR:
-
Insulin resistance index of homeostasis model assessment
- BMI:
-
Body mass index
- RCS:
-
Restricted cubic spline
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- WC:
-
Waist circumference
- HC:
-
Hipline circumference
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- TG:
-
Triglycerides
- TC:
-
Serum total cholesterol
- DM:
-
Diabetes mellitus
- HTN:
-
Hypertension
- UA:
-
Uric acid
- Cr:
-
Creatinine
References
Tsatsoulis, A., Mantzaris, M. D., Bellou, S. & Andrikoula, M. Insulin resistance: An adaptive mechanism becomes maladaptive in the current environment—an evolutionary perspective. Metabolism. 62(5), 622–633 (2013).
Reaven, G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler. Thromb. Vasc. Biol. 32(8), 1754–1759 (2012).
Laakso, M. & Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol. 10(5), 293–302 (2014).
Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 26(2), 19–39 (2005).
Reaven, G. M. Pathophysiology of insulin resistance in human disease. Physiol. Rev. 75(3), 473–486 (1995).
Ormazabal, V. et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 17(1), 122 (2018).
Bornfeldt, K. E. & Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 14(5), 575–585 (2011).
Townsend, R. R. et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension 66(3), 698–722 (2015).
Chirinos, J. A., Segers, P., Hughes, T. & Townsend, R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 74(9), 1237–1263 (2019).
Ohkuma, T. et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: An individual participant data meta-analysis. Hypertension 69(6), 1045–1052 (2017).
Kitahara, T. et al. Impact of brachial-ankle pulse wave velocity and ankle-brachial blood pressure index on mortality in hemodialysis patients. Am. J. Kidney Dis. 46(4), 688–696 (2005).
Li, Y.-H., Sheu, W.H.-H. & Lee, I. T. Use of the ankle-brachial index combined with the percentage of mean arterial pressure at the ankle to improve prediction of all-cause mortality in type 2 diabetes mellitus: An observational study. Cardiovasc. Diabetol. 19(1), 173 (2020).
Orchard, T. J., Chang, Y.-F., Ferrell, R. E., Petro, N. & Ellis, D. E. Nephropathy in type 1 diabetes: A manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int. 62(3), 963–970 (2002).
Schofield, C. J. & Sutherland, C. Disordered insulin secretion in the development of insulin resistance and Type 2 diabetes. Diabet. Med. 29(8), 972–979 (2012).
Nakanishi, N., Shiraishi, T. & Wada, M. Brachial-ankle pulse wave velocity and metabolic syndrome in a Japanese population: The Minoh study. Hypertens. Res. 28(2), 125–131 (2005).
Lee, S. B. et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc. Diabetol. 17(1), 41 (2018).
Wang, S. et al. Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: A real-world single-centre study. Cardiovasc. Diabetol. 20(1), 82 (2021).
Galvin, P. et al. A simple method for quantitation of insulin sensitivity and insulin release from an intravenous glucose tolerance test. Diabet Med. 9(10), 921–928 (1992).
Zhao, S. et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: The Northern Shanghai Study. Cardiovasc. Diabetol. 18(1), 95 (2019).
Chang, D. R. et al. The ratio and difference of urine protein-to-creatinine ratio and albumin-to-creatinine ratio facilitate risk prediction of all-cause mortality. Sci. Rep. 11(1), 7851 (2021).
Fu, S., Lin, Y., Luo, L. & Ye, P. Relationship between Central Arterial Stiffness and Insulin Resistance in Chinese Community-Dwelling Population without Diabetes Mellitus. Int. J. Endocrinol. 2017, 1073919 (2017).
Li, M. et al. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: The China H-type Hypertension Registry Study. Cardiovasc. Diabetol. 19(1), 139 (2020).
Su, Y. et al. Triglyceride glucose index associated with arterial stiffness in Chinese community-dwelling elderly. Front. Cardiovasc. Med. 8, 737899 (2021).
Won, K.-B. et al. Relationship of insulin resistance estimated by triglyceride glucose index to arterial stiffness. Lipids Health Dis. 17(1), 268 (2018).
Cox, A. J., Hsu, F.-C., Carr, J. J., Freedman, B. I. & Bowden, D. W. Glomerular filtration rate and albuminuria predict mortality independently from coronary artery calcified plaque in the Diabetes Heart Study. Cardiovasc. Diabetol. 12, 68 (2013).
Potier, L. et al. Relationship between cardiac microvascular dysfunction measured with 82Rubidium-PET and albuminuria in patients with diabetes mellitus. Cardiovasc. Diabetol. 17(1), 11 (2018).
Matsushita, K. et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 3(7), 514–525 (2015).
Jackson, C. E. et al. Albuminuria in chronic heart failure: Prevalence and prognostic importance. Lancet 374(9689), 543–550 (2009).
Coward, R. J. M. et al. The human glomerular podocyte is a novel target for insulin action. Diabetes 54(11), 3095–3102 (2005).
Welsh, G. I. et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 12(4), 329–340 (2010).
Sarafidis, P. A. & Ruilope, L. M. Insulin resistance, hyperinsulinemia, and renal injury: Mechanisms and implications. Am. J. Nephrol. 26(3), 232–244 (2006).
Taniguchi, A. et al. C-reactive protein and insulin resistance in non-obese Japanese type 2 diabetic patients. Metabolism 51(12), 1578–1581 (2002).
Mykkänen, L. et al. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: The insulin resistance atherosclerosis study. Diabetes 47(5), 793–800 (1998).
Chen, Z. et al. A nonlinear associations of metabolic score for insulin resistance index with incident diabetes: A retrospective Chinese cohort study. Front. Clin. Diabetes Healthc. 3, 1101276 (2022).
Wang, Z., Li, W., Li, J. & Liu, N. The nonlinear correlation between a novel metabolic score for insulin resistance and subclinical myocardial injury in the general population. Front. Endocrinol. 13, 889379 (2022).
Wu, S. et al. Association between triglyceride-glucose index and risk of arterial stiffness: A cohort study. Cardiovasc. Diabetol. 20(1), 146 (2021).
Xie, Q. et al. Association between MetS-IR and prediabetes risk and sex differences: A cohort study based on the Chinese population. Front. Endocrinol. 14, 1175988 (2023).
Cai, X. et al. Dose-response associations of metabolic score for insulin resistance index with nonalcoholic fatty liver disease among a nonobese chinese population: Retrospective evidence from a population-based cohort study. Dis. Mark. 2022, 4930355 (2022).
Acknowledgements
We are grateful to all survey members who contributed to the work and the participants in the study.
Funding
This study was supported by the National Key R&D Program of China (2020YFC2008900), the National Defense Science and Technology Innovation Special Zone Project (223-CXCY-N101-07-18-01), and the Key Projects of Logistics Scientific Research Project of Chinese PLA (19BJZ30).
Author information
Authors and Affiliations
Contributions
C.M., B.C., and L.Z. designed the study, analyzed the data, drafted the manuscript, and revised the manuscript. S.C. and B.Q. contributed to the collection and analysis of the data. J.S., Q.B. and M.L. assisted in manuscript revision. S.Z. and Y.C. assisted in the data analysis. S.W., G.X. and P.Z. suggested research directions and revised the manuscript. C.M., B.C., and L.Z. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, C., Cheng, B., Zhou, L. et al. Association between insulin resistance and vascular damage in an adult population in China: a cross-sectional study. Sci Rep 14, 18472 (2024). https://doi.org/10.1038/s41598-024-69338-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69338-y
- Springer Nature Limited