Abstract
Background
Homeostasis model assessment for insulin resistance (HOMA-IR) is a biomarker for type 2 diabetes mellitus (T2DM). However, the role of HOMA-IR in the non-diabetic is unclear. This study aimed to determine whether IR measured HOMA-IR value is associated with new onset diabetes as well as vascular disease and can be used as an early predictor for diabetes and vascular diseases in non-diabetic participants.
Methods
From a prospective community-based cohort of 10,030 individuals, 4314 individuals younger than 65 years and without diabetes were enrolled and divided into three groups by baseline HOMA-IR tertiles: low (n = 1454), moderate (n = 1414), and high (n = 1446). The primary outcome was new onset T2DM. Secondary outcomes were chronic kidney disease (CKD) and a composite of coronary artery disease, myocardial infarction, and stroke as macrovascular events.
Results
The mean age was 51 years. The prevalence of hypertension and cholesterol and HbA1c were higher in the high HOMA-IR group. New onset T2DM (5.8%) and CKD (12.2%) incidence in the high HOMA-IR group was higher than that in the others. The prevalence of macrovascular events did not differ among groups. High-HOMA-IR was an independent risk factor for new onset T2DM (odds ratio 1.86 [1.17–2.96]; p = 0.01) and CKD (1.49 [1.12–1.98]; p = 0.01).
Conclusions
High HOMA-IR was an early predictor of new onset T2DM and CKD, regardless of HbA1c in non-diabetic individuals. Further research on the specific cut off value will be needed.
Similar content being viewed by others
Background
Diabetes mellitus (DM) and its complications are increasing, and the related medical cost is becoming a socio-economic burden worldwide [1]. DM is also closely related to other metabolic diseases, such as dyslipidaemia and fatty liver [2]. Current treatments target individual metabolic diseases, such as hypertension, dyslipidaemia, and diabetes, and are focused on maintenance therapy that prevents disease deterioration rather than preventing and managing the root of the disease. Therefore, besides the high medical costs, the prevalence of metabolic diseases and the associated mortality rates are increasing. To prevent diabetes and its complications, it is important to identify high-risk populations and prevent the disease’s onset in the early and reversible phases.
Insulin resistance (IR), a state of impaired biological response to normal circulating levels of insulin, represents an early pathophysiology of diabetes progression and is associated with micro and macrovascular diseases, as reported by cross-sectional epidemiologic studies [3,4,5,6]. Recent short-term observational studies have also suggested that IR may be a risk factor for the development of DM [7]. However, it is not yet clear whether IR is a consequence of diabetes and diabetic complication or a factor leading to it. In this study, we investigated whether IR measured using the homeostatic model assessment for insulin resistance (HOMA-IR) value is associated with new onset diabetes as well as vascular disease and can be used as an early predictor for diabetes and vascular diseases in non-diabetic participants from a large prospective community-based cohort.
Methods
Study populations
Data were collected from the Ansan (urban) and Ansung (rural) prospective community-based cohort studies. These studies are part of the Korean Health and Genome Study (KoGES), which is conducted by the Korea Centers for Disease Control and Prevention (Republic of Korea) as a government-funded epidemiological survey to investigate trends in chronic non-communicable diseases and their associated risk factors. From June 2001 to January 2003, adults aged 40–69 years residing in Ansan and Ansung were enrolled. The cohort included a total of 10,030 adults (5018 from Ansung and 5020 from Ansan) who underwent health examination at the Korea University Ansan Hospital and Ajou University Medical Center [8]. The distributions of age and gender were similar to those in the general population. Surveys were conducted every two years through clinical examinations and questionnaires, and a total of six follow-ups were conducted until 2014.
Our study only included participants from the cohort who were under the age of 65 and did not have diabetes. The following were exclusion criteria: age over 65 years at first visit, HbA1c test performed only once at first visit, unavailable HOMA-IR value or diagnosis of diabetes at baseline as follows: 1) HbA1c ≥ 6.5% [9] and 2) taking DM medication at the time of the first visit.
Covariates
All covariates were based on the time of the first visit and included clinical and biochemical data. Clinical data, such as age, gender, smoking status, hypertension, dyslipidaemia, previous myocardial infarction (MI), previous heart failure, and previous chronic kidney disease (CKD), were obtained using standardised questionnaires by a trained interviewer. Biochemical data, including HbA1c, fasting blood glucose and insulin, lipid profile, and biomarkers reflecting systemic inflammatory status (high sensitivity C-reactive protein), were also obtained, as previously described [10]. Blood samples were obtained after an overnight fast of at least 8 h, and HbA1c levels were measured using high-performance liquid chromatography (Variant II; BioRad Laboratories, Hercules, CA, USA). The glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease equation at each visit [11]. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2).
The fasting insulin and glucose values were used to calculate the values for HOMA-IR, homeostasis model of assessment–β-cell (HOMA–β-cell), and quantitative insulin sensitivity check index (QUICKI) [12, 13]. The subjects were divided into three groups by HOMA-IR value tertiles; the first tertile was 1.37, and the second tertile was 1.84.
Outcome definition
The primary outcome was new onset DM. New onset DM was diagnosed based on A1C criteria (HbA1C ≥ 6.5%) or taking DM medication during follow up. Secondary outcomes were defined as CKD and macrovascular events. CKD was defined as a creatinine clearance rate of < 60 mL/min/1.73 m2. Subjects with CKD at baseline were excluded from the survival analysis. A macrovascular event was defined as a composite of coronary artery disease, MI, and ischemic stroke reported through a questionnaire [3].
Statistical analysis
All statistical analyses were performed using the SPSS (version 24.0; IBM Corp., Armonk, NY, USA) and R (version 3.1.10; the R Foundation for Statistical Computing, Vienna, Austria) software. Categorical variables are presented as frequencies with percentages and were compared between groups using the chi-square test or Fisher’s exact test. Continuous variables are presented as either mean (± standard deviation) and were compared between groups using one-way analysis of variance.
The cumulative incidences of primary outcomes were compared between groups using the Kaplan–Meier method, with the log rank test. The odds ratios (ORs) and confidence intervals (CIs) for primary and secondary outcomes according to HOMA-IR groups were estimated using multivariable logistic regression analysis after adjustment of variables. Model 1 adjusted clinical factors, such as age, sex, current smoking, hypertension, dyslipidaemia, MI, heart failure, CKD, and HbA1c. Model 2 additionally adjusted laboratory variables, such as high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, C-reactive protein, HOMA-β-cell, GFR, and BMI. Statistical significance was considered at p-value < 0.05.
Ethical considerations
The Institutional Review Board of Gwangju Institute of Science and Technology (South Korea) approved the study protocol (IRB No. 20200414-EX-01-02). All research procedures were performed in accordance with the relevant guidelines and regulations. All participants volunteered for the Ansan and Ansung studies and provided written informed consent.
Results
Baseline characteristics
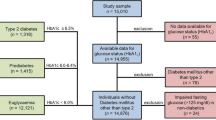
Among the 10,030 individuals in the study cohort, 1784 individuals who only tested for HbA1c once were excluded. In addition, participants were excluded if they were > 65 years old at baseline (n = 983) or diagnosed with DM at baseline (n = 801) and without an HOMA-IR value (n = 2148). Finally, 4314 individuals were enrolled in this study (Fig. 1). The participants were divided into low (n = 1454), moderate (n = 1414), and high (n = 1446) HOMA-IR groups based on HOMA-IR value tertiles.
The subjects were followed for a median interval of 9.9 years (interquartile range: 9.1–10.0 years). Clinical and biochemical baseline characteristics of the HOMA-IR groups are presented in Table 1. The mean age was 51 years in all HOMA-IR groups, and the proportions of men in the low, moderate, and high HOMA-IR groups were 56.6, 47.3, and 45.4%, respectively. The prevalence of hypertension was higher in the high HOMA-IR group (16.7%) than in the moderate (10.7%) and low (8.8%) HOMA-IR groups, but the prevalence of dyslipidaemia, previous MI, previous heart failure, and CKD were not significantly different among the HOMA-IR groups.
The mean values of HOMA-IR were 1.02, 1.51, and 2.43 and those of HOMA-β-cell were 90, 102, and 122, respectively, in the low, moderate, and high groups. The high HOMA-IR group had a higher mean HbA1c, total cholesterol, triglyceride, and LDL-cholesterol and lower HDL-cholesterol than the other groups, although all values were within normal ranges. In contrast, C-reactive protein and baseline GFR values were not different among the three groups.
Primary outcomes for the HOMA-IR groups
The primary outcome was defined as new onset DM, and the secondary outcomes were defined as CKD and macrovascular events. New onset DM was observed in a total of 164 participants, and more participants were newly diagnosed with diabetes in the high HOMA-IR group (n = 84, 5.8%) than in the other groups (p-value < 0.001). CKD was observed in a total of 425 participants, and more participants were diagnosed in the high HOMA-IR group (n = 173, 12.2%) than in the other groups (p-value = 0.002). On the contrary, macrovascular events, including coronary artery disease, MI, and ischemic stroke, were observed in a total of 102 participants, and there was no significant difference in the incidence of macrovascular events among the HOMA-IR groups (Table 2, Fig. 2).
HOMA-IR as an independent predictor of new onset diabetes and CKD
Table 3 shows the OR for primary and secondary outcomes in the HOMA-IR groups. After adjusting for clinical risk factors in model 1, high HOMA-IR was found to be a marginal risk factor for new onset DM (OR: 1.42, 95% CI: 0.95–2.14, p-value = 0.09). Additionally, after adjusting for baseline laboratory variables and BMI in model 2, high HOMA-IR was a significant risk factor for the development of DM (OR: 1.86, 95% CI: 1.17–2.96, p-value = 0.01). The baseline HbA1c level was also a significant risk factor for new onset diabetes. The results for CKD were similar to those for new onset diabetes. In model 1, the OR for newly diagnosed CKD in the high HOMA-IR group was 1.42 (95% CI: 1.10–1.84, p-value = 0.01) and in model 2, the OR for newly diagnosed CKD in the high HOMA-IR group was 1.49 (95% CI: 1.12–1.98, p-value = 0.01). In contrast, the HbA1c level was not a risk factor for the development of CKD. There was no significant difference in ORs between high HOMA-IR and HbA1c values for macrovascular events in both models. BMI, as an indicator of metabolic disease, is also a significant risk factor for new onset DM but not vascular events (Table S1 of Additional file 1).
Changes in HOMA-IR and HOMA-β-cell values between the baseline and last visit were calculated (Fig. S1 of Additional file 1). The HOMA-IR value was significantly increased in the low and moderate HOMA-IR groups compared to that in the high HOMA-IR group. HOMA-β-cell decreased over time, regardless of the group. The decrease in HOMA-β-cell was significantly higher in the high HOMA-IR group than in the low and moderate HOMA-IR groups.
Discussion
Analysis of a non-diabetic middle-aged population from a large prospective, community-based cohort with a long term follow up revealed a significantly higher prevalence of new onset diabetes and CKD in the high HOMA-IR group than in the other groups. High baseline HOMA-IR was an independent risk factor for both new onset diabetes and CKD regardless of the HbA1c level. However, there was no association between high HOMA-IR and macrovascular events.
Many previous studies have shown the relationship between IR and diabetes [3, 4, 6]. IR refers to reduced responsiveness to insulin in tissues that take up glucose, such as liver, skeletal muscle, and adipose tissue [14, 15]. In compensation for IR, the synthesis of insulin in β cells increases and hyperinsulinemia occurs, leading to impaired glucose disposal [15]. Type 2 diabetes mellitus (T2DM) is induced when there is a combination of insufficiencies in β cell mass and function to meet the demands of IR. A high serum glucose level inhibits the proliferation and de-differentiation of β cells through a process called “glucotoxicity,” which gradually leads to reduced insulin secretion [16]. In the high HOMA-IR group, the HOMA-β-cell value for β cell function decreased more than that in the other groups in our study. Given that individuals with T2DM have a β cell mass and function occasionally preserved within normal range in the early period of T2DM progression, β cell mass and function insufficiencies were relative rather than absolute. Although an insufficient β cell mass is essential for the development of T2DM [17], it is difficult to accurately measure β cell mass in living people, and insulin secretion capacities widely vary; therefore, β cell mass has limited use as a biomarker for new onset diabetes.
In contrast, IR is commonly observed in most T2DM patients and in individuals with impaired glucose tolerance. IR is reportedly the strongest predictor of T2DM, and diabetes can be prevented by improving IR [5]. IR begins from the very early stage of diabetes and can thus be used as an early biomarker to estimate the risk of new onset diabetes. A recent Saku study [4] assessed IR and diabetes in 2209 non-diabetic patients. Changes in HOMA-IR were measured in the non-diabetic patients and showed that the incidence of T2DM was high when the changes in HOMA-IR were moderate or high. The Saku study also showed that IR had a strong impact on the development of diabetes. This finding is line with our study. Our study showed a significant relationship between high HOMA-IR and new onset diabetes, even in non-diabetic patients from a larger prospective community-based cohort with ten years of follow up.
BMI is a metabolic disease parameter and is associated with IR and diabetes [18]. In our study, BMI was found to be a significant risk factor for new onset DM but not vascular events. Because BMI is calculated based only on height and weight, it does not seem to represent metabolic status more sensitively than other parameters, such as visceral fat and waist-to-hip ratio [19,20,21]. Vascular disease is also directly affected by factors other than metabolic disease, such as high blood pressure.
Another important finding of the present study was that high HOMA-IR value was found to be an independent risk factor for CKD among non-diabetic individuals, whereas HbA1c was not. Various studies have been conducted on the relationship between CKD and IR [22,23,24,25]. CKD, itself characterised by a low-grade inflammatory state, can cause IR and vice versa. CKD and IR adversely affect each other, accelerating the deterioration of renal function [23]. There are several mechanisms that have been suggested to underly the relationship between CKD and IR, one of which is hyperinsulinemia, which increases oxidative stress, protein glycosylation oxidation, and lipid peroxidation [5]. Hyperinsulinemia causes glomerular hyperfiltration, endothelial dysfunction, and increased vascular permeability. IR with oxidative stress and inflammation is thought to play roles in microalbuminuria development and kidney function impairment [5]. In addition to hyperinsulinemia, inappropriate activation of the renin-angiotensin-aldosterone system may cause renal insufficiency [26]. Eventually, IR can lead to glomerulosclerosis and tubulointerstitial injury. A 3-year prospective cohort study with 7200 patients showed that the incidence of CKD and rate of decrease of eGFR were higher in the high HOMA-IR group with metabolic syndrome [27]. Our study also showed that high HOMA-IR was an independent risk factor for CKD after adjusting for multiple risk factors, including HbA1c and baseline GFR. HbA1c was not a risk factor for CKD in our study. This might be due to the fact that the HbA1c value in our study was within the normal range, unlike the 7% HbA1c standard value for predicting microvascular complications in the UKPDS study [26].
The incidence of macrovascular events did not differ among the HOMA-IR groups. Some studies have reported a relationship between IR and cardiovascular events. However, it is difficult to directly compare these with our study, since most of these previous studies have a cross-sectional design and involved a few participants or participants who already had atherosclerosis identified as a high-risk factor [22, 27,28,29]. The clinical significance of IR for cardiovascular disease may more likely be as a factor accelerating disease progression in patients with certain risk factors, such as CKD, rather than as an independent risk factor [22, 29]. Another issue with our study was that it focused on a population that was relatively young and healthy, and thus, the risk of cardiovascular events was very low. Major vascular complications begin to develop about 10 years after diabetes diagnosis. Follow-up of the cohort in the present study is still ongoing; thus, we hope to observe very long-term cardiovascular events and influence of high baseline HOMA-IR value.
There are several limitations to this study. First, IR was evaluated using only HOMA-IR. The gold standard for evaluating IR is the hyperinsulinemia-euglycemic glucose clamp technique [12], but it is clinically difficult to implement and even more difficult to apply in large-scale cohort studies. In contrast, HOMA-IR is widely used to measure IR and has yielded reliable results in many studies [28, 29]. Second, clinical data were obtained through standardised questionnaires by a trained interviewer. However, the incidence of macrovascular events in this relatively healthy cohort was lower than that among people with diabetes. Large cohort studies routinely use standardised questionnaires, and the incidence of macrovascular events in our Korean cohort was similar to that in other ethnic groups without diabetes [30]. Third, the absence of data regarding other microvascular events, such as retinopathy, could be a limitation, although the expected incidences of end-stage DM-related microvascular events in our cohort are very low, as the participants did not have diabetes at baseline. It is difficult to infer the effect of specific medication on clinical events because of the lack of medication data. As mentioned above, participants included in this analysis had a very low incidence of risk factors; thus, the effect of drugs in our cohort is expected to be insignificant.
Conclusions
Our findings indicate that high baseline HOMA-IR has a significant relationship with the development of T2DM and CKD and is an independent risk factor for both new onset T2DM and CKD, regardless of the HbA1c level in a healthy middle-age population. However, we found no association between high HOMA-IR and macrovascular events. This suggests that HOMA-IR measurement can be used as a biomarker to identify people at risk for T2DM development. Further studies are needed to define a HOMA-IR cut off value as a new onset T2DM prediction marker and determine its association with macrovascular disease through long-term follow up.
Availability of data and materials
The corresponding author, Ji-Young Jang, may provide the data that back up the conclusions of this study upon request. Researchers who wish to use the integrate dataset can access the KoGES epidemiological data online sharing system (https://nih.go.kr/ko/main/contents.do?menuNo=300564). A guidebook for using the integrated dataset is also available in the online sharing system.
Abbreviations
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CKD:
-
chronic kidney disease
- CRP:
-
C-reactive protein
- GFR:
-
Glomerular filtration rate
- HbA1c:
-
Glycated haemoglobin
- HDL:
-
High-density lipoprotein
- HOMA-IR:
-
Homeostasis model assessment for insulin resistance
- MI:
-
Myocardial infarction
- LDL:
-
Low-density lipoprotein
- QUICKI:
-
Quantitative insulin sensitivity check index
- T2DM:
-
Type 2 diabetes mellitus
- UKPDS:
-
The UK Prospective Diabetes Study
References
Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21.
Calanna S, Scicali R, Di Pino A, Knop FK, Piro S, Rabuazzo AM, et al. Lipid and liver abnormalities in haemoglobin A1c-defined prediabetes and type 2 diabetes. Nutr Metab Cardiovasc Dis. 2014;24:670–6.
Tang Q, Li X, Song P, Xu L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: developments in research and prospects for the future. Drug Discov Ther. 2015;9:380–5.
Morimoto A, Tatsumi Y, Soyano F, Miyamatsu N, Sonoda N, Godai K, et al. Increase in homeostasis model assessment of insulin resistance (HOMA-IR) had a strong impact on the development of type 2 diabetes in Japanese individuals with impaired insulin secretion: the Saku study. PLoS One. 2014;9:e105827.
Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, Whelton PK, et al. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol. 2003;14:469–77.
Baek JH, Kim H, Kim KY, Jung J. Insulin resistance and the risk of diabetes and dysglycemia in Korean general adult population. Diabetes Metab J. 2018;42:296–307.
Khalili D, Khayamzadeh M, Kohansal K, Ahanchi NS, Hasheminia M, Hadaegh F, et al. Are HOMA-IR and HOMA-B good predictors for diabetes and pre-diabetes subtypes? BMC Endocr Disord. 2023;23:1–9.
Kim Y, Han BG, Ko GESg. Cohort profile: the Korean genome and epidemiology study (KoGES). Consortium Int J Epidemiol. 2017;46:e20.
American Diabetes Association; 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl. 1):S13–28. https://doi.org/10.2337/dc19-S002.
Lim S, Jang HC, Lee HK, Kim KC, Park C, Cho NH. A rural-urban comparison of the characteristics of the metabolic syndrome by gender in Korea: the Korean health and genome study (KHGS). J Endocrinol Investig. 2016;29:313–9.
Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968–83.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10.
Kosmas CE, Silverio D, Tsomidou C, Salcedo MD, Montan PD, Guzman E. The impact of insulin resistance and chronic kidney disease on inflammation and cardiovascular disease. Clin Med Insights Endocrinol Diabetes. 2018;11:1179551418792257.
Freeman AM. Pennings N. StatPearls Treasure Island (FL): Insulin Resistance; 2019.
Weir GC, Bonner WS. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53:S16–21.
Weir GC, Gaglia J, Bonner-Weir S. Inadequate β-cell mass is essential for the pathogenesis of type 2 diabetes. Lancet Diabetes Endocrinol. 2020;8:249–56.
Diniz M, Beleigoli AMR, Schmidt MI, Duncan BB, Ribeiro ALP, Vidigal PG, et al. Homeostasis model assessment of insulin resistance (HOMA-IR) and metabolic syndrome at baseline of a multicentric Brazilian cohort: ELSA-Brasil study. Cad Saude Publica. 2020;36:e00072120.
Scicali R, Rosenbaum D, Di Pino A, Giral P, Cluzel P, Redheuil A, et al. An increased waist-to-hip ratio is a key determinant of atherosclerotic burden in overweight subjects. Acta Diabetol. 2018;55:741–9.
Fernstrom M, Fernberg U, Hurtig-Wennlof A. Insulin resistance (HOMA-IR) and body fat (%) are associated to low intake of fruit and vegetables in Swedish, young adults: the cross-sectional lifestyle, biomarkers and atherosclerosis study. BMC Nutr. 2019;5:15.
Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, et al. Am J Physiol Endocrinol Metab. 2010;299:E506–15.
Schrauben SJ, Jepson C, Hsu JY, Wilson FP, Zhang X, Lash JP, et al. Insulin resistance and chronic kidney disease progression, cardiovascular events, and death: findings from the chronic renal insufficiency cohort study. BMC Nephrol. 2019;20:60.
Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311:F1087–108.
Liao MT, Sung CC, Hung KC, Wu CC, Lo L, Lu KC. Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol. 2012;2012:691369.
Hu Y, Shi LX, Zhang Q, Peng NC. Increased risk of chronic kidney diseases in patients with metabolic syndrome: a 3-year prospective cohort study. Curr Med Sci. 2019;39:204–10.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Scicali R, Giral P, Gallo A, Di Pino A, Rabuazzo AM, Purrello F, et al. HbA1c increase is associated with higher coronary and peripheral atherosclerotic burden in non diabetic patients. Atherosclerosis. 2016;255:102–8.
Mossmann M, Wainstein MV, Goncalves SC, Wainstein RV, Gravina GL, Sangalli M, et al. HOMA-IR is associated with significant angiographic coronary artery disease in non-diabetic, non-obese individuals: a cross-sectional study. Diabetol Metab Syndr. 2015;7:100.
Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30:318–24.
Barengo NC, Teuschl Y, Moltchanov V, Laatikainen T, Jousilahti P, Tuomilehto J. Coronary heart disease incidence and mortality, and all-cause mortality among diabetic and non-diabetic people according to their smoking behavior in Finland. Tob Induc Dis. 2017;2(15):12. https://doi.org/10.1186/s12971-017-0113-3.
Acknowledgements
Not applicable
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education (2016R1A6A3A04010466, 2020R1C1C1004999 to C.M.O.). No funder/sponsor had any role in the design and conduct of the study.
Author information
Authors and Affiliations
Contributions
Ji-Yong Jang, MD had full access to all data in the study and take responsibility for data integrity and data analysis accuracy. Jibeom Lee and MH Kim were equally responsible for drafting of the manuscript. All authors have participated in editing the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All participants volunteered for the Ansan and Ansung studies and provided written informed consent. The study protocol was approved by the institutional review board of the Gwangju Institute of Science and Technology (IRB No. 20200414-EX-01-02). All the research procedures were performed in accordance with guidelines and regulations.
Consent for publication
I, the undersigned, give my consent for the publication of identifiable details within the text to be published in Clinical Diabetes and Endocrinology.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Odds ratios for body mass index in multivariate analysis of primary and secondary outcomes. Figure S1. Progressive change of HOMA-IR and HOMA-beta cell function during follow-up (* indicated p <0.001).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, J., Kim, Mh., Jang, JY. et al. Assessment HOMA as a predictor for new onset diabetes mellitus and diabetic complications in non-diabetic adults: a KoGES prospective cohort study. Clin Diabetes Endocrinol 9, 7 (2023). https://doi.org/10.1186/s40842-023-00156-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40842-023-00156-3