Abstract
Pyrethroid bednets treated with the synergist piperonyl butoxide (PBO) offer the possibility of improved vector control in mosquito populations with metabolic resistance. In 2017–2019, we conducted a large-scale, cluster-randomised trial (LLINEUP) to evaluate long-lasting insecticidal nets (LLINs) treated with a pyrethroid insecticide plus PBO (PBO LLINs), as compared to conventional, pyrethroid-only LLINs across 104 health sub-districts (HSDs) in Uganda. In LLINEUP, and similar trials in Tanzania, PBO LLINs were found to provide greater protection against malaria than conventional LLINs, reducing parasitaemia and vector density. In the LLINEUP trial, we conducted cross-sectional household entomological surveys at baseline and then every 6 months for two years, which we use here to investigate longitudinal changes in mosquito infection rate and genetic markers of resistance. Overall, 5395 female Anopheles mosquitoes were collected from 5046 households. The proportion of mosquitoes infected (PCR-positive) with Plasmodium falciparum did not change significantly over time, while infection with non-falciparum malaria decreased in An. gambiae s.s., but not An. funestus. The frequency of genetic markers associated with pyrethroid resistance increased significantly over time, but the rate of change was not different between the two LLIN types. The knock-down resistance (kdr) mutation Vgsc-995S declined over time as Vgsc-995F, the alternative resistance mutation at this codon, increased. Vgsc-995F appears to be spreading into Uganda. Distribution of LLINs in Uganda was previously found to be associated with reductions in parasite prevalence and vector density, but here we show that the proportion of infective mosquitoes remained stable across both PBO and non-PBO LLINs, suggesting that the potential for transmission persisted. The increased frequency of markers of pyrethroid resistance indicates that LLIN distribution favoured the evolution of resistance within local vectors and highlights the potential benefits of resistance management strategies.
Trial registration: This study is registered with ISRCTN, ISRCTN17516395. Registered 14 February 2017, http://www.isrctn.com/ISRCTN17516395.
Similar content being viewed by others
Introduction
Long-lasting insecticidal nets (LLINs) are the principal tool for malaria control in Africa and were the major driver of the decline in malaria mortality between 2000 and 20151,2. Growing concerns over the rapid spread of resistance to the pyrethroid insecticides with which the nets aretreated3,4 have been partially assuaged by promising trials of new generation LLINs treated with the synergist piperonyl butoxide (PBO)5,6,7,8, which inhibits the activity of cytochrome P450s, one of the major causes of insecticide resistance in malaria vectors. The LLIN Evaluation in Uganda Project (LLINEUP, Trial registration: ISRCTN17516395, 14/02/2017), was a cluster randomised trial of conventional LLINs (PermaNet 2.0 and Olyset) and PBO-LLINs (PermaNet 3.0 and Olyset Plus). The trial found that parasite prevalence, adjusted for baseline, was 20% lower in children from communities receiving PBO LLINs than in those receiving conventional LLINs, for up to 25 months post-LLIN distribution (parasite prevalence ratio 0.80 [95% CI 0.69–0.93], P = 0.0048)5. Vector abundance, again adjusted for baseline, was 73% lower in PBO communities than in non-PBO communities (Vector density ratio 0.27 [95% CI 0.21–0.36], P < 0.0001)5 .
In this paper we report on the frequency of Plasmodium infection and molecular markers of insecticide resistance over the course of the trial, testing four hypotheses about the change over time after bednet deployment. First, regarding parasite infection, we tested the following hypothesis:
H1
The previously reported decline in human malaria rates after LLIN distribution leads to a decline in Plasmodium infection in Anopheles mosquitoes.
Insecticide resistance is widespread in malaria vectors in Uganda9,10,11,12,13, and has been implicated in the limited epidemiological impact of vector control campaigns14,15,16. The World Health Organization (WHO) has identified insecticide resistance management (IRM) as a key strategy to prolong the effective lifespan of insecticide-based interventions17. A central tenet of IRM programmes is that use of insecticides within the public health sector selects for resistance and therefore the rational deployment of resistance management tools through rotations, mixtures and mosaics etc. can delay the onset of resistance. However, it is unclear whether the application of insecticides for public health is the primary driver of insecticide resistance, or whether use of insecticides for agriculture contributes. Demonstrating the role of the former is important for developing IRM strategies. The primary vector of malaria in Uganda is An. gambiae s.s10, and it is the species for which the largest array of molecular markers are available. We thus focused our analyses on this species.
In a survey of 5,200 households conducted from March to June 2017, in advance of LLIN distribution, only 14.8% of residents lived in a household with adequate LLIN coverage, with nets having been delivered during the previous Universal Coverage Campaign (UCC) between 29 to 53 months earlier and therefore being towards the end of their useful lifespan18. Following the UCC conducted as part of the LLINEUP study, 96.7% of surveyed households had an LLIN with 70.8% having adequate coverage. These values remained far in excess of baseline throughout the trial5. The scale and diverse ecologies over which the LLINEUP trial was conducted afforded us the opportunity to test three hypotheses regarding molecular markers of insecticide resistance in this species. First, distribution of new bednets, with resulting increasing bednet coverage and renewal of the active ingredient concentration, should lead to an increase in pyrethroid resistance. We therefore tested the following hypothesis:
H2
The use of pyrethroid insecticides on LLINs selects for an increase in frequency of molecular markers of pyrethroid resistance over the course of the intervention.
A key assumption of IRM strategies is that the use of insecticide synergists, such as PBO, should retard the evolution of resistance. We examine whether there was any evidence for differences in resistance marker frequency changes between intervention arms receiving conventional LLINs and those receiving PBO-LLINs. A priori we proposed the following hypothesis:
H3
The rate of change in frequency in cytochrome P450 resistance markers will differ in clusters which received PBO-LLINs vs conventional LLINs.
One of the resistance markers we genotype, the Cyp6aap-Dup1 haplotype, is more strongly associated with resistance to Class II pyrethroids (including deltamethrin and α-cypermethrin) than to Class I pyrethroids (permethrin)12. We therefore propose:
H4
The rate of increase in the Cyp6aap-Dup1; ZZB-TE, Cyp6p4-236M triple mutant haplotype will be greater in clusters which received conventional deltamethrin LLINs (PermaNet 2.0) relative to clusters which received conventional permethrin LLINs (Olyset).
Methods
Household selection, mosquito collection and processing.
Full details of the sampling procedures are given in10,19, Fig. 1. In brief, in each round of surveys, 50 households were randomly selected from an enumeration list of households in each of the 104 health sub-districts (HSDs) for the cross-sectional community surveys. Of those 50 households, 10 were randomly selected to take part in the entomology surveys, giving a maximum of 1040 households for entomological surveillance. In the final round of surveys (25 months post net distribution) it was only possible to survey 90 of the 104 HSDs due to restrictions resulting from the COVID-19 pandemic. Mosquitoes were collected using Prokopack aspirators20 and DNA extractions were carried out on the head and thorax using Nexttec Biotechnologie DNA extraction plates (Nexttec Biotechnologie GmbH, Hilgertshausen, Germany), and these extractions were used for all subsequent molecular assays. Anopheles gambiae s.l. and An. funestus s.l. mosquitoes were identified to species level by PCR21,22, with 46 out of 5442 samples failing to amplify and therefore being discarded from further analysis. Malaria infections in An. gambiae s.l. and An. funestus s.l. were detected by a P. falciparum, P. vivax, P. ovale and P. malariae Taqman assay23. Since only the head and thorax were used for DNA extraction, rather than the gut, presence of Plasmodium is likely to indicate sporozoite infection. Even if there is slight error in our PCR-based estimates, our use of the results is not to obtain an exact sporozoite rate, but rather to compare infection before and after intervention, for which the PCR-based measure is suitable.
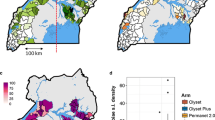
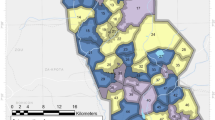
Map of mosquito collection locations within the LLINEUP cluster-randomised control trial. Intervention arm is indicated by colour with the 14 clusters which were omitted during final collection round (25 months post LLIN distribution) indicated with hatched shading. Figure created using QGIS v 3.32 (http://qgis.osgeo.org).
The baseline entomological screening identified that An. gambiae s.s. was the primary vector across the study area10 and we therefore prioritised molecular work on this species. An. gambiae s.s. females were screened for pyrethroid resistance mutations; Vgsc-995F, Vgsc-995S, Vgsc-1570Y, Cyp6aap-Dup1 triple mutant haplotype (consisting of Cyp6aap-Dup1 itself, Cyp6p4-236M and the transposable element insertion ZZB), Cyp4J5-43F and Coeae1d following standard protocols12,24,25,26,27. The 2La chromosome inversion karyotype of An. gambiae s.s. specimens was assessed by PCR28.
Data analysis was carried out in R statistical software version 4.1.329 (www.r-project.org). Analysis of sporozoite infection and molecular marker frequency data used General Linear Models with logit link function for a binomial dependent variable, implemented in the R package glmmTMB30. Collections were conducted over 5 rounds, but mosquito numbers dropped sharply after the LLINs were distributed (Supplementary Fig. 1), as previously reported5,7, with a notable increase by round 5 (Table 1). Thus, few mosquito samples were available for evaluation in intermediate rounds, particularly in communities that received PBO LLINs. We therefore ran the analysis of genotype frequencies both using data from across all rounds, and focusing on comparisons between baseline and Round 5 (Table 3 and5).
For each of our four hypotheses, we identified the model best suited to test the significance of the term of interest. We kept the total number of terms as small as possible, to avoid over-fitting, while still controlling for the main potential confounding factors: “Location”, “Arm” and “HSD”. The terms in these models are: “Plasmodium” (binomial variable of presence/absence of Plasmodium infection according to PCR), “Marker” (binomial variable of presence/absence of mutant form at the haplotype level), “Round” (intervention round 1–5 coded as numeric variable), “Round(1vs5)” (intervention rounds 1 and 5 only, coded as factor), “Location” (East or West Uganda), “Arm” (PBO or non-PBO (conventional) LLIN as treated), “HSD” (health sub-district).
We tested H1 by obtaining the P value (anova function with chi-squared test in R) associated with removing the “Round” term in the following model: Plasmodium ~ Round + Location + Arm + (1|HSD). We tested H2 by considering the effect of removing the “Round” term in the following two models: Model 1—Glmm (Marker ~ Round + Location + Arm + (1|HSD)); Model 2—Glmm (Marker ~ Round(1vs5) + Location + Arm + (1|HSD)). The first model takes all five round, while the second compares only round 1 against round 5. Similarly, we tested H3 by considering the effect of removing the Round: Arm interaction term in the following two models: Model 3—Glmm (Marker ~ Round + Location + Arm + Round: Arm + (1|HSD)); Model 4—Glmm (Marker ~ Round(1vs5) + Location + Arm + Round(1vs5):Arm + (1|HSD)). Finally, we tested H4 by taking the P value of the Round: Insecticide interaction term in the following models, including only samples from the conventional nets arm: Model 5—Glmm (Marker ~ Round + Location + Insecticide + Round: Insecticide + (1|HSD)); Model 6—Glmm (Marker ~ Round(1vs5) + Location + Insecticide + Round(1vs5): Insecticide + (1|HSD)). The P-values of relevance to our hypotheses were corrected for multiple testing by controlling the false discovery rate (FDR)31.
Spatiotemporal variation in marker frequencies was analysed by fitting a Bayesian geostatistical model to the frequencies of each marker observed in the mosquitoes collected from each household in rounds 1–532. The numbers of each allele present in each sample were assumed to follow a binomial distribution, with a mean probability modelled as a spatiotemporal random effect depending on latitude, longitude and round. Spatial autocorrelation was modelled using a Gaussian Markov random field and temporal autocorrelation was an autoregressive model of order 1. Models were fitted using the R-INLA package33,34. The script to produce these models and map the outcome are available at https://github.com/vigg-lstm/llineup-genotyping/tree/main/INLA_mapping.
Anopheles household abundance data were reported previously in the main trial papers5,7 with molecular data from baseline collections published in the baseline entomology paper10. All data from the 6, 12, 18 and 25 month collection rounds and associated analyses are unique to this manuscript. All collection data and analytical routines are available on GitHub (https://github.com/vigg-lstm/llineup-genotyping).
Ethics approval and consent to participate
The study was approved by the Ugandan National Council for Science and Technology (UNCST Ref HS 2176), Makerere University School of Medicine Research & Ethics Committee (SOMREC 2016-133), London School of Hygiene & Tropical Medicine Ethics Committee (LSHTM Ref 12019), and the Liverpool School of Tropical Medicine (Ref 16-072), which sponsored the study. All research was performed in accordance with relevant guidelines/regulations. Written informed consent to participate in the study was obtained by the head of household (or their designate) for all participating households.
Results
Overall, 5395 female Anopheles mosquitoes were collected (resting indoor collections using aspirators) from 5046 households in the five surveys (Table 1), including 1797 in round 1 (baseline survey), 423 in round 2 (6-months post-LLIN distribution), 755 in round 3 (12-months), 1093 in round 4 (18-months) and 1327 in round 5 (25-months). At baseline, the prevalence of Plasmodium falciparum infection in An. gambiae s.s. was 5.6% (72/1284) and in An. funestus was 3.5% (15/432). Other Plasmodium species (P. vivax, P. ovale, and P. malariae) were detected less commonly in both An. gambiae s.s. (1.2%, 16/1284) and An. funestus (1.4%, 6/432)10. No Plasmodium infections were detected in An. arabiensis (Supplementary Table 1) and they were excluded from further analysis.
H1
The previously reported decline in human malaria rates after LLIN distribution leads to a resulting decline in Plasmodium infection in Anopheles mosquitoes.
The prevalence of P. falciparum infection in Anopheles mosquitoes did not change significantly over the course of the study (P = 0.074 and P = 0.86 for An. gambiae and An. funestus respectively). In contrast, the combined prevalence of other Plasmodium species decreased significantly in An. gambiae (P = 0.018), but not An. funestus (P = 0.36; Fig. 2; Table 2), although after correction for multiple testing, the false discovery rate was found to be slightly higher than 5% (Q = 0.073). There was no evidence for a significant interaction between collection round and study arm (ie, a difference in the slope of infection prevalence over time between intervention arms) for either P. falciparum or other non-falciparum Plasmodium.
Plasmodium infection prevalence in An. gambiae and An. funestus. Point prevalence estimate is shown with associated 95%CIs. Data were collected simultaneously but are plotted offset for ease of viewing. Round 1 was the baseline collection with follow up rounds at approximately six-monthly intervals (see text).
The resistance associated variant Vgsc-1570Y was not found in any specimen and is not discussed further. There was no significant association between any of the genotypic markers screened and infection with P. falciparum or the other non-falciparum Plasmodium (Supplementary Fig. 2).
H2
The use of pyrethroid insecticides on LLINs selects for an increase in frequency of molecular markers of pyrethroid resistance over the course of the intervention.
Five pyrethroid resistance markers showed a significant change in frequency (Vgsc-995F; Vgsc-995S; Cyp6aap-Dup1; ZZB-TE, Cyp6p4-236 M) (Fig. 3 and Table 3), either over the course of study follow-up (P = 1 × 10–9, P = 3 × 10–9, P = 9 × 10–12, P = 4 × 10–5, P = 7 × 10–5, for the five markers respectively; Table 3 Model 1), or when comparing frequencies at baseline with those from the final collection round (P = 3 × 10–9, P = 1 × 10–8/, P = 7 × 10–12, P = 3 × 10–6, P = 9 × 10–6; Table 3 Model 2). A sixth marker (Cyp4j5-43F) also showed significant changes by raw P-values over the course of the study (P = 0.03), but the Q values after FDR correction were around 0.06, indicating that accepting these as significant would lead to an overall rate of 6% false positives among our significant P-values. The statistical significance of the change for Cyp4j5-43F is therefore ambiguous. Cyp6aap-Dup1; ZZB-TE, Cyp6p4-236 M are in near full linkage disequilibrium on a triple-mutant haplotype12, so subsequent analyses focused on Cyp6p4-236 M as representative of this haplotype. No significant changes in frequency for 2La or Coeae1d resistance markers were observed (respectively, P = 0.1 and P = 0.63 over the course of the study, P = 0.37 and P = 0.75 when comparing rounds 1 and 5). In the 25 months following LLIN distribution, all resistance markers increased significantly in frequency, except for Vgsc-995S which decreased significantly (dropping from 94% in both conventional and PBO-LLINs to 79% (PBO) and 91% (conventional) frequency). These increases are consistent with the hypothesis that LLINs treated with pyrethroids (a public health intervention) exert selective pressure and drive pyrethroid resistance. The Vgsc-995S mutation is in negative linkage with Vgsc-995F, with relatively few wild-type alleles in the population10. Thus, the decrease in Vgsc-995S is an expected consequence of the increase in Vgsc-995F.
Insecticide resistance marker prevalence in Anopheles gambiae s.s. across the baseline and four post LLIN distribution collection rounds. Markers in green external boxes show significant increases (see Table 3) over the course of the trial, red boxes indicate significant decreases (Table 3). Dashed line indicates significant change observed only when GLMM included all five collection points.
Mapping the change in frequency of Vgsc-995F suggested that this mutation gradually spread from the North-West of the country over the 2-year study period (Fig. 4). In contrast, the increase in frequency of Cyp4j5-43F occurred in all areas, and particularly in the Southern regions. At baseline, the Cyp6p4-236M (triple-mutant haplotype) was already present at high frequency (> 65%) across the study area, and the greatest increases in frequency of this allele were in the North-East, where the baseline frequencies were lowest.
H3
The rate of change in frequency of cytochrome P450 resistance markers will differ in clusters which received PBO-LLINs vs conventional LLINs.
Cyp4j5-43F and Cyp6p4-236M (triple-mutant haplotype) showed contrasting trends in frequency (Fig. 3). For Cyp4j5-43F, the interaction between collection round and LLIN arm was significant by raw P-value, with false discovery rates close to 0.05 both over the course of the study (P = 0.025, Q = 0.056, Table 3 Model 3) and when comparing pre-LLIN distribution frequencies with the final collection round (P = 0.014, Q = 0.036, Table 3 Model 4). The direction of the interaction indicates that the rate of increase in this P450-mediated resistance mechanism is, perhaps counter-intuitively, higher in clusters which received PBO-LLINs. Conversely there was no evidence for a significant interaction between round and intervention arm for the triple haplotype marker, Cyp6p4-236M.
H4
The rate of increase in the triple mutant haplotype will be greater in clusters which receive deltamethrin LLINs (PermaNet 2.0) relative to clusters which receive permethrin LLINs (Olyset).
There was no evidence that rates of change in frequency of the triple-mutant haplotype were different between clusters receiving conventional LLINs treated with either permethrin or deltamethrin (P = 0.55 over the course of the study, P = 0.34 between rounds 1 and 5; Table 3 models 5 and 6).
Discussion
To investigate the impact of LLINs on the emergence and spread of insecticide resistance in Uganda, we evaluated Anopheles mosquitoes collected from 48 districts over the 2-year follow-up period of the LLINEUP trial. We found that although parasite prevalence and vector density decreased in both study arms following the distribution of LLINs5, the rate of infection with Plasmodium falciparum in sampled mosquitoes did not change significantly over the study, while rates of infection with other Plasmodium species decreased significantly in An. gambiae, but not An. funestus. Since the number of mosquitoes collected following LLIN distribution was low, it is possible that the lack of significance is a result of low statistical power. However, in the case of An. funestus, there was no suggestion of an overall decrease in infection over time (the model coefficient was positive). The reduction in parasite prevalence5 and mosquito numbers does not appear to have markedly reduced infection rates in the Anopheles vectors analysed. In the LLINEUP trial, the reduction in parasite prevalence was observed in children aged 2–10 years. It is possible that parasitaemia in older children and adults persisted, thus providing opportunities for mosquitoes to take infected blood meals from this reservoir of older residents. Increased mosquito mortality, inferred from reduced vector collections, is expected to result in a younger mosquito population, leading to a smaller proportion of mosquitoes living long enough to become infective. However, increased adult mortality does not necessarily result in a younger age distribution if it causes a population decline35, and mosquito infection rate may therefore be unaffected.
We found no evidence of association between resistance marker genotype and infection status. These results contrast to those from a previous study which detected an association between Vgsc-995S genotype and infection, consistent with the hypothesis that mosquitoes carrying resistant alleles had increased longevity and were therefore more likely to survive the parasite extrinsic incubation period36. One difference that could explain these results is that overall Vgsc-995 and Cyp6p4-236M mutant frequencies were high in our study, with very few wild-type alleles found in the population. There may therefore have been too few fully susceptible individuals to detect an effect of resistance on Plasmodium infection.
There was clear evidence of increases in genotypic markers of pyrethroid resistance over the study. Although it not possible to randomise resistance between trial arms, and thus we cannot exclude the possibility that marker frequency would have increased even in the absence of LLINs, we consider the considerable scale-up of LLIN coverage to be the most likely explanation for the results as the mosquitoes were collected from HSDs representing ≈40% of the surface area of Uganda, encompassing marked differences in ecology, altitude, socio-economic status of communities etc.5. Previous work to correlate LLIN distributions with changes in resistance have yielded contrasting results, arguably due to their reliance upon inherently noisy resistance phenotyping approaches37. Our use of genotypic markers provides a metric that can be accurately quantified, reducing the noise in the statistical analysis. The significant increase in Vgsc-995F frequency at the expense of the alternative Vgsc-995S allele suggests that the former mutation is gradually replacing the latter, and mapping of allele frequencies indicates that this replacement is centred in the North-West of the country. Vgsc-995F is a predominantly West- and Central-African allele, and thus its higher frequency in the North-Western part of Uganda is consistent with a gradual spread eastwards. The Vgsc-995F was first observed in An. gambiae s.s. from the region in 201238. At this time the Vgsc-995S variant was near fixation39 and analyses suggested that the Vgsc-995S mutation was older40 and more strongly associated with DDT rather than pyrethroid resistance41,42. The increase in frequency of the Vgsc-995F mutation provides additional support to our contention that LLINs are a major driver of pyrethroid resistance.
PBO LLINs have been promoted as an intervention that can overcome, at least in part, cytochrome P450-mediated resistance. A priori we would have predicted that mosquito populations sampled from communities that received PBO LLINs would show a decrease in P450-resistance markers relative to conventional LLINs. However whilst there were contrasting patterns observed between different P450 marker systems, the only evidence of a significant change was a relative increase in frequency of the Cyp4j5-43F marker. It may be that incomplete suppression of cytochrome P450s does not remove the selective pressure to upregulate this form of resistance, but rather escalates an arms race in which mosquitoes further upregulate cytochrome P450-mediated resistance to overcome the suppressive effects of PBO.
Conclusions
The large-scale deployment of LLINs in Uganda in 2017–2019 was associated with an increase in the frequency of genotypic markers of pyrethroid resistance, but not in the frequency of P. falciparum infection in mosquitoes. The reduction in malaria prevalence resulting from the LLIN distribution campaign was therefore likely the result of decreased mosquito numbers, rather than fewer infective mosquitoes. The increase in resistance allele frequency suggests that public health interventions, such as LLINs, can apply selective pressure which drives the evolution of insecticide resistance, supporting the need for resistance management strategies.
Data accessibility and Benefit-Sharing
The datasets reported herein are publicly available in the GitHub repository https://github.com/vigg-lstm/llineup-genotyping, as are the scripts used in the analysis of these data. GPS coordinates of the households used in the study have been removed for data protection. Benefits Generated: A research collaboration was developed with scientists from the country providing genetic samples, all collaborators are included as co-authors, the results of research have been shared with the provider communities and the broader scientific community, and the research addresses a priority concern, in this case the impact of PBO-LLINs on the evolution of resistance to insecticides in malaria mosquitoes.
References
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015).
Geneva. Global report on insecticide resistance in malaria vectors: 2010–2016 (2016).
Glunt, K. D. et al. Empirical and theoretical investigation into the potential impacts of insecticide resistance on the effectiveness of insecticide-treated bed nets. Evolut. Appl. 11, 431–441 (2018).
Hemingway, J. et al. Averting a malaria disaster: will insecticide resistance derail malaria control?. Lancet 387, 1785–1788 (2016).
Maiteki-Sebuguzi, C. et al. Effect of long-lasting insecticidal nets with and without piperonyl butoxide on malaria indicators in Uganda (LLINEUP): Final results of a cluster-randomised trial embedded in a national distribution campaign. Lancet Infect. Dis. 23, 247–258 (2022).
Protopopoff, N. et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: A cluster, randomised controlled, two-by-two factorial design trial. Lancet 391, 1577–1588 (2018).
Staedke, S. G. et al. Effect of long-lasting insecticidal nets with and without piperonyl butoxide on malaria indicators in Uganda (LLINEUP): A pragmatic, cluster-randomised trial embedded in a national LLIN distribution campaign. Lancet 395, 1292–1303 (2020).
Tiono, A. B. et al. Efficacy of Olyset Duo, a bednet containing pyriproxyfen and permethrin, versus a permethrin-only net against clinical malaria in an area with highly pyrethroid-resistant vectors in rural Burkina Faso: A cluster-randomised controlled trial. Lancet 392, 569–580 (2018).
Abeku, T. A. et al. Insecticide resistance patterns in Uganda and the effect of indoor residual spraying with bendiocarb on kdr L1014S frequencies in Anopheles gambiae ss. Malar. J. 16, 1–11 (2017).
Lynd, A. et al. LLIN Evaluation in Uganda Project (LLINEUP): A cross-sectional survey of species diversity and insecticide resistance in 48 districts of Uganda. Parasites Vectors 12, 94 (2019).
Mawejje, H. D. et al. Insecticide resistance monitoring of field-collected Anopheles gambiae sl populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Med. Vet. Entomol. 27, 276–283 (2013).
Njoroge, H. et al. Identification of a rapidly-spreading triple mutant for high-level metabolic insecticide resistance in Anopheles gambiae provides a real-time molecular diagnostic for antimalarial intervention deployment. Mol. Ecol. 31, 4307–4318 (2022).
Okia, M. et al. Insecticide resistance status of the malaria mosquitoes: Anopheles gambiae and Anopheles funestus in eastern and northern Uganda. Malar. J. 17, 1–12 (2018).
Epstein, A. et al. Resurgence of malaria in Uganda despite sustained indoor residual spraying and repeated long lasting insecticidal net distributions. PLOS Glob. Public Health 2, e0000676 (2022).
Katureebe, A. et al. Measures of malaria burden after long-lasting insecticidal net distribution and indoor residual spraying at three sites in Uganda: A prospective observational study. PLoS Med. 13, e1002167 (2016).
Kigozi, R. et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLOS ONE 7, e42857 (2012).
Geneva: World Health Organization. Global plan for insecticide resistance management in malaria vectors (GPIRM) (2012).
Gonahasa, S. et al. LLIN Evaluation in Uganda Project (LLINEUP): factors associated with ownership and use of long-lasting insecticidal nets in Uganda: A cross-sectional survey of 48 districts. Malar. J. 17, 1–14 (2018).
Staedke, S. G. et al. LLIN Evaluation in Uganda Project (LLINEUP)–Impact of long-lasting insecticidal nets with, and without, piperonyl butoxide on malaria indicators in Uganda: Study protocol for a cluster-randomised trial. Trials 20, 1–13 (2019).
Vazquez-Prokopec, G. M., Galvin, W. A., Kelly, R. & Kitron, U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J. Med. Entomol. 46, 1256–1259 (2009).
Koekemoer, L. L., Kamau, L., Hunt, R. H. & Coetzee, M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J Trop. Med. Hyg. 66, 804–811 (2002).
Santolamazza, F. et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 7, 163 (2008).
Bass, C. et al. PCR-based detection of Plasmodium in Anopheles mosquitoes: a comparison of a new high-throughput assay with existing methods. Malar. J. 7, 1–9 (2008).
Bass, C. et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar. J. 6, 1–14 (2007).
Jones, C. M. et al. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc. Natl. Acad. Sci. 109, 6614–6619 (2012).
Lynd, A. et al. Insecticide resistance in Anopheles gambiae from the northern Democratic Republic of Congo, with extreme knockdown resistance (kdr) mutation frequencies revealed by a new diagnostic assay. Malar. J. 17, 412 (2018).
Weetman, D. et al. Candidate-gene based GWAS identifies reproducible DNA markers for metabolic pyrethroid resistance from standing genetic variation in East African Anopheles gambiae. Sci. Rep. 8, 2920 (2018).
White, B. J. et al. Molecular karyotyping of the 2La inversion in Anopheles gambiae. Am. J. Trop. Med. Hyg. 76, 334–339 (2007).
R Core Team. R: A Language and Environment for Statistical Computing (2015).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B-Methodol. 57, 289–300 (1995).
Hancock, P. A. et al. Modelling spatiotemporal trends in the frequency of genetic mutations conferring insecticide target-site resistance in African mosquito malaria vector species. BMC Biol. 20, 1–17 (2022).
Lindgren, F., Rue, H. & Lindström, J. An explicit link between Gaussian fields and Gaussian Markov random fields: the stochastic partial differential equation approach. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 73, 423–498 (2011).
Rue, H., Martino, S. & Chopin, N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Soc. Series b (Stat. Methodol.) 71, 319–392 (2009).
Abrams, P. A. Does increased mortality favor the evolution of more rapid senescence. Evolution 47, 877–887 (1993).
Kabula, B. et al. A significant association between deltamethrin resistance, Plasmodium falciparum infection and the Vgsc-1014S resistance mutation in Anopheles gambiae highlights the epidemiological importance of resistance markers. Malar. J. 15, 1–5 (2016).
Implications of Insecticide Resistance Consortium. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: Trends in pyrethroid resistance during a WHO-coordinated multi-country prospective study. Parasites Vectors 11, 550 (2018).
Ochomo, E. et al. Presence of the knockdown resistance mutation, Vgsc-1014F in Anopheles gambiae and An arabiensis in western Kenya. Parasites Vectors 8, 616 (2015).
Mathias, D. K. et al. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae ss and phenotypic variability in susceptibility to insecticides in Western Kenya. Malaria J. 10, 1–13 (2011).
Lynd, A. et al. Field, genetic, and modeling approaches show strong positive selection acting upon an insecticide resistance mutation in Anopheles gambiae ss. Mol. Biol. Evol. 27, 1117–1125 (2010).
Donnelly, M. J., Isaacs, A. T. & Weetman, D. Identification, validation, and application of molecular diagnostics for insecticide resistance in malaria vectors. Trends Parasitol. 32, 197–206 (2016).
Reimer, L. J. et al. An unusual distribution of the kdr gene among populations of Anopheles gambiae on the island of Bioko Equatorial Guinea. Insect Mol. Biol. 14, 683–688 (2005).
Acknowledgements
The authors would like to thank all the staff and administration at IDRC for their considerable efforts in facilitating this project. In particular Martin Mugote, Violet Tuhaise, Jackson Asiimwe and Kilama Maxwell Kilama for their supervision of field activities; Peter Mutungi, and Simon Peter Kigozi and Geoff Lavoy for their considerable assistance in data management; Lillian Taaka, Christine Nabirye and Nicholas Wendo for assistance in logistics and procurement; Susan Nayiga, Isiko Joseph, Erias Muyanda and Henry Opolot for coordination and support. We are extremely grateful to the entomology field workers for collecting and identifying mosquitoes, and to the community survey teams and team leaders who facilitated their work. We would also like to acknowledge and thank the members of the Uganda National Malaria Control Program and the Liverpool School of Tropical Medicine for logistical and other support provided as we carried out these surveys and the district health, administrative, and political leadership teams for all their support and guidance during community entry in the 48 districts of the study area. We thank Dr David Weetman for comments on an earlier version of the manuscript.
Funding
This project was supported by The Against Malaria Foundation. Additional funding was provided by Award Number R01AI116811 from the National Institutes of Allergy and Infectious Diseases (NIAID) and a Wellcome Trust MSc Fellowship in Public Health and Tropical Medicine (Oruni-203511/Z/16/Z). The content of the manuscript is solely the responsibility of the authors.
Author information
Authors and Affiliations
Contributions
S.G.S., M.R.K., G.D., and M.J.D. conceived of the study, with input from J.O., A.Y., and J.H. S.G.S., G.D., and M.J.D. developed the procedures and drafted the protocol with M.R.K. and J.H. A.L. led the data collection in the field, with oversight from S.G., and support from C.M.S., J.O., M.R.K., A.K., M.K. and S.G.S. A.L. and A.O. performed all laboratory analyses. A.L., E.R.L., D.Mc.D., P.H., E.K. and M.J.D. managed the data and led the data analysis. A.L., E.R.L. and M.J.D. interpreted the data and drafted the manuscript, with input from SGS. All authors reviewed the manuscript and gave permission for publication. M.J.D., the corresponding author, had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lynd, A., Gonahasa, S., Staedke, S.G. et al. LLIN Evaluation in Uganda Project (LLINEUP)–effects of a vector control trial on Plasmodium infection prevalence and genotypic markers of insecticide resistance in Anopheles vectors from 48 districts of Uganda. Sci Rep 14, 14488 (2024). https://doi.org/10.1038/s41598-024-65050-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65050-z
- Springer Nature Limited