Abstract
Background
The success of malaria vector control is threatened by widespread pyrethroid insecticide resistance. However, the extent to which insecticide resistance impacts transmission is unclear. The objective of this study was to examine the association between the DDT/pyrethroid knockdown resistance mutation Vgsc-1014S, commonly termed kdr, and infection with Plasmodium falciparum sporozoites in Anopheles gambiae.
Methods
WHO standard methods were used to characterize susceptibility of wild female mosquitoes to 0.05 % deltamethrin. PCR-based molecular diagnostics were used to identify mosquitoes to species and to genotype at the Vgsc-L1014S locus. ELISAs were used to detect the presence of P. falciparum sporozoites and for blood meal identification.
Results
Anopheles mosquitoes were resistant to deltamethrin with mortality rates of 77.7 % [95 % CI 74.9–80.3 %]. Of 545 mosquitoes genotyped 96.5 % were A. gambiae s.s. and 3.5 % were Anopheles arabiensis. The Vgsc-1014S mutation was detected in both species. Both species were predominantly anthropophagic. In A. gambiae s.s., Vgsc-L1014S genotype was significantly associated with deltamethrin resistance (χ2 = 11.2; p < 0.001). The P. falciparum sporozoite infection rate was 4.2 %. There was a significant association between the presence of sporozoites and Vgsc-L1014S genotype in A. gambiae s.s. (χ2 = 4.94; p = 0.026).
Conclusions
One marker, Vgsc-1014S, was associated with insecticide resistance and P. falciparum infection in wild-caught mixed aged populations of A. gambiae s.s. thereby showing how resistance may directly impact transmission.
Similar content being viewed by others
Background
Malaria vector control in Africa relies on insecticide-treated nets (ITNs) and indoor residual spraying (IRS) which in turn depends on vector susceptibility. Great progress has been made during the past decade with the distribution of approximately 427 million ITNs, enough to cover over 80 % of the 840 million people at risk of malaria in sub-Saharan Africa [1]. Similarly, the number of people protected by IRS in the region increased from 13 million in 2005 to 81 million in 2010, accounting for approximately 11 % of the at risk population [1]. However, the widespread development of insecticide resistance in malaria vectors (reviewed in [2]) draws into question the sustainability of the gains achieved through insecticide-based malaria vector control.
Quantifying the insecticide susceptibility of vector populations is advocated as an integral part of malaria control campaigns [3]. Whilst there are numerous mortality and knockdown phenotyping assays there is only limited evidence of their associations with epidemiological outcomes; moreover they are subject to environment-induced error [4]. A number of researchers have advocated for the use of molecular diagnostics for resistance monitoring, as they should permit early detection of insecticide resistance. However, as discussed previously [5], there are barriers to the adoption of molecular markers by malaria control programmes for monitoring and evaluation beyond the obvious financial and logistical constraints. One of these major barriers is an observed variability in the power of molecular markers to predict results from phenotyping assays; partially a result of low power when markers approach fixation and/or the impact of environmental conditions on phenotype (discussed in [6]). Given the questionable epidemiological value of extant phenotyping tests, groups are developing improved assays [7]. However by associating known resistance markers directly with transmission indices (sporozoite infection) it is possible to remove the intermediate step of associating molecular resistance markers with an arbitrary resistance phenotype that may have no simple epidemiological interpretation.
Methods
Study site
The study was carried out in Zeneti village (5° 13′ S; 38° 39′ E) in Muheza district, northeast Tanzania. Malaria is intense and perennial with transmission peaking after the rainy season in May and June [8].
Mosquito collections and processing
Mosquitoes were sampled between (November 2012 and May 2013). Indoor resting collections were used to obtain live females for deltamethrin susceptibility testing and pyrethrum spray catches (PSC) were used for mosquitoes that were collected for blood meal analysis. Collections were conducted between 06:00 and 09:00 h from 10–15 randomly selected houses. Live mosquitoes collected for susceptibility testing were provided with 10 % glucose solution and transported to the field insectary. Mosquitoes were sorted and morphologically identified to species. The blood meals from freshly fed females collected by PSC were squashed onto Whatman filter paper and dried. The remaining carcasses were stored individually over desiccant for laboratory processing.
Insecticide susceptibility tests
Insecticide susceptibility tests were carried out using World Health Organization (WHO) tubes and standard operating procedures [9] with four replicates of 15–25 wild adult female mosquitoes per tube. Mosquitoes were exposed to papers impregnated with the WHO recommended discriminating concentration of 0.05 % deltamethrin prepared by University Sains Malasyia. Deltamethrin was chosen because it has a long history of use in the treatment of bed nets in the area, and Anopheles gambiae s.l. have developed resistance to the insecticide [10, 11]. The controls were exposed to paper impregnated with silicone oil. Mortality was scored after a 24-h holding period, during which time mosquitoes were provided with a 10 % sugar solution.
Detection of Plasmodium falciparum infection and blood meal sources
Plasmodium falciparum sporozoite infections [12] and blood meal origins [13] were detected by ELISA (Enzyme Linked Immunosorbent Assay). Only the heads and thoraces were used to detect P. falciparum sporozoite infections. The blood ELISA screened for human, bovine and caprine antibodies.
Molecular species identification and detection of point mutations in the voltage-gated sodium channel (Vgsc)
Molecular screening was conducted on mosquitoes collected for susceptibility tests/infection study and for detection of blood meal origin. The remaining body parts of these mosquitoes, not used in the ELISAs above, were transferred to a 96-well plate and DNA extracted using the DNeasy Blood and Tissue kit (Qiagen). The standard PCR protocol to identify A. gambiae s.l. species was used [14] together with a Taqman assay to screen for the 1014S mutation in the voltage-gated sodium channel [15].
Ethical consideration
The study was approved by the Kilimanjaro Christian Medical College Research Ethics and Review Committee (CRERC) and the Medical Research Coordinating Committee (MRCC). The communities, where sampling was done, were informed on the project and informed consent sought from the local authorities within each community. Informed consent was also sought from the households where mosquito sampling was carried out.
Results
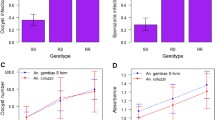
A total of 948 adult female A. gambiae mosquitoes were exposed to the standard WHO dosage of deltamethrin; an additional 295 females were exposed to control papers in order to detect excess mortality due to exposure conditions. All control animals survived and are not considered further. Overall mortality rates of Anopheles mosquitoes 24 h post-exposure were 77.7 % (95 % CI 74.9–80.3 %). A total of 545 mosquitoes phenotyped by WHO tube test for deltamethrin susceptibility were successfully genotyped to species and for the Vgsc-L1014S point mutation. Out of these, 526 (96.5 %) were identified as A. gambiae s.s. and 19 (3.5 %) were Anopheles arabiensis. The Vgsc-1014S point mutation was detected in both A. arabiensis and A. gambiae s.s. The mutation was found at an allelic frequency of 0.45 (95 % CI 0.41–0.48) in A. gambiae s.s. and 0.32 (95 % CI 0.19–0.47) in A. arabiensis. The frequency of Vgsc-1014S in A. arabiensis and A. gambiae s.s. did not differ significantly (Fisher’s exact test p = 0.195) but given small number of A. arabiensis screened this analysis has limited power. Given their far larger sample size all subsequent analyses refer solely to A. gambiae s.s.. There was a significant association between deltamethrin resistance phenotype and Vgsc-L1014S genotype (Table 1).
Of 526 A. gambiae s.s. mosquitoes for which a deltamethrin susceptibility phenotype and a Vgsc-L1014S genotype were obtained, 22 (4.2 %) were P. falciparum sporozoite positive. Despite this relatively low overall infection rate, there were a significantly higher proportion of sporozoite positives in Vgsc-1014S homozygotes relative to wildtype (Table 1). Heterozygotes showed an intermediate sporozoite infection rate relative to wild type and kdr homozygotes suggesting that the kdr allele may not be not fully recessive for the infection phenotype (Table 1).
Blood meal analyses of the mosquitoes sampled using PSC revealed high degrees of anthropophagy (Total n = 575; A. gambiae s.s. 95.4 %, A. arabiensis 71.3 %) although as expected A. gambiae s.s. had a significantly higher proportion of human blood meals (χ2 = 34.11; p < 0.001). Anthropophagy in A. gambiae s.s. was not associated with the presence of the Vgsc-1014S allele (Table 1).
Discussion
The basis for selecting this geographical location were previous records of high levels of pyrethroid resistance [16]. Resistance may have remained high in this area due to the cumulative impact of continued ITNs use and scale-up. Insecticide resistance associated with scaling up of ITN and IRS interventions has been reported from across sub-Saharan Africa [17–20].
This study which, unusually, used female A. gambiae s.s. mosquitoes of mixed age, demonstrated a significant association between deltamethrin resistance phenotype and Vgsc-L1014S genotype. Studies based upon heterologous expression of the Drosophila melanogaster Vgsc in Xenopus eggs suggest that the 1014S mutation is maximally effective against, and may have been selected by, DDT [21]. Therefore, perhaps unsurprisingly, field studies showing an association between resistance to class II pyrethroids and 1014S genotype are few [6, 11]. In some instances this may, as noted in western Tanzania, reflect that the 1014S allele is close to fixation [22]. However in another study in neighbouring Uganda no genotype:phenotype association was observed even with the marker at an intermediate frequency and where associations were demonstrated for DDT and Class I pyrethroids [6, 23]. Given that additional resistance associated variants may accumulate on a kdr background [24] it may be that 1014S is a marker for two different haplotypes with differing resistance profiles, in Uganda/western Tanzania and eastern Tanzania (this study).
A key component of the entomological inoculation rate is the proportion of mosquitoes that are infected. There was a significant association between Vgsc-L1014S genotype and infection with P. falciparum in A. gambiae s.s. with infection rates in Vgsc-1014S homozygotes over three time higher that in wildtype females. The overall P. falciparum infection rate of 4.2 % was lower than that previously recorded in this area 7 % [25]. This may in part reflect a reduction in infection rates resulting from sub-lethal insecticide exposure. In a recent experimental study from Uganda sub-lethal exposure to deltamethrin resulted in reductions in both the proportion and intensity of infection of P. falciparum infection in A. gambiae [26]. However, in this study Vgsc-1014S homozygotes had sporozoite rates of 8 % suggesting that the mutation may in essence counteract the effects of sub-lethal exposure on infection.
For both deltamethrin susceptibility and sporozoite infection Vgsc-L1014S heterozygotes exhibit phenotypes that were intermediate between the extremes observed in both homozygote groups. This implies that for these phenotypes the Vgsc-1014S allele is not fully recessive as modelling studies on the Vgsc-1014F allele have previously suggested [27].
It was not possible resolve whether the association between infection and Vgsc-1014S genotype is a result of an increase in daily survival which means Vgsc-1014S carriers are more likely to survive the parasite extrinsic incubation period or a more direct Vgsc-1014S—P. falciparum interaction. Experimental studies may support this latter hypothesis as, in the absence of insecticidal pressure, higher sporozoite rates were recorded in Vgsc-1014F carrying An. gambiae s.s. compared to wild type [28]. RNAi silencing of a serine protease, ClipC9, in linkage disequilibrium with Vgsc-1014F resulted in reduced P. falciparum infection, suggesting a possible mechanism [29].
Conclusions
This study demonstrated that in a Tanzanian population of A. gambiae s.s., phenotypic resistance to deltamethrin and infection with P. falciparum is significantly associated with the Vgsc-1014S point mutation. Whilst, fortunately there is no evidence of catastrophic failure of vector control programmes associated with the presence of this or other Vgsc mutations, these data suggest that in areas with high IRS and/or LLINs coverage Vgsc-1014S is potentially impacting control efforts by increasing P. falciparum infection rates. It is hoped that these data are a spur to malaria control programmes to integrate molecular resistance monitoring into their monitoring and evaluation programmes.
References
WHO. World Malaria Report 2014. Geneva: World Health Organization; 2014:242.
Ranson H, Lissenden N. Insecticide resistance in African anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–96.
WHO-GMP. Global plan for insecticide resistance management in malaria vectors (GPIRM). Geneva: World Health Organization; 2012.
Kleinschmidt I, Mnzava AP, Kafy HT, Mbogo C, Bashir AI, Bigoga J, et al. Design of a study to determine the impact of insecticide resistance on malaria vector control: a multi-country investigation. Malar J. 2015;14:13.
Weetman D, Donnelly MJ. Evolution of insecticide resistance diagnostics in malaria vectors. Trans R Soc Trop Med Hyg. 2015;109:291–3.
Donnelly MJ, Isaacs A, Weetman D. Identification, validation, and application of molecular diagnostics for insecticide resistance in malaria vectors. Trends Parasitol. 2016;32:197–206.
Bagi J, Grisales N, Corkill R, Morgan JC, N’Fale S, Brogdon WG, et al. When a discriminating dose assay is not enough: measuring the intensity of insecticide resistance in malaria vectors. Malar J. 2015;14:210.
Maxwell CA, Chambo W, Mwaimu M, Magogo F, Carneiro IA, Curtis CF. Variation of malaria transmission and morbidity with altitude in Tanzania and with introduction of alphacypermethrin treated nets. Malar J. 2003;2:28.
WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Geneva: World Health Organization; 2012.
Kabula B, Tungu P, Matowo J, Kitau J, Mweya C, Emidi B, et al. Susceptibility status of malaria vectors to insecticides commonly used for malaria control in Tanzania. Trop Med Int Health. 2012;17:742–50.
Kabula B, Kisinza W, Tungu P, Ndege C, Batengana B, Kollo D, et al. Co-occurrence and distribution of east (L1014S) and west (L1014F) African knock-down resistance in Anopheles gambiae sensu lato population of Tanzania. Trop Med Int Health. 2014;19:331–41.
Wirtz RA, Burkot TR, Graves PM, Andre RG. Field evaluation of enzyme linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera, Culicidae) from Papua New Guinea. J Med Entomol. 1987;24:433–7.
Beier JC, Perkins PV, Wirtz RA, Koros J, Diggs D, Gargan TP, et al. Bloodmeal identification by direct enzyme-linked immunosorbent-assay (ELISA), tested on Anopheles (Diptera, culicidae) in Kenya. J Med Entomol. 1988;25:9–16.
Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111.
Kabula B, Tungu P, Malima R, Rowland M, Minja J, Wililo R, et al. Distribution and spread of pyrethroid and DDT resistance among the Anopheles gambiae complex in Tanzania. Med Vet Entomol. 2014;28:244–52.
Stump AD, Atieli FK, Vulule JM, Besansky NJ. Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. Am J Trop Med Hyg. 2004;70:591–6.
Sharp B, Ridl F, Govender D, Kuklinski J, Kleinschmidt I. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malar J. 2007;6:52.
Czeher C, Labbo R, Arzika I, Duchemin J-B. Evidence of increasing leu-phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar J. 2008;7:189.
Ndiath MO, Sougoufara S, Gaye A, Mazenot C, Konate L, Faye O, et al. Resistance to DDT and pyrethroids and increased kdr mutation frequency in An. gambiae after the implementation of permethrin-treated nets in Senegal. PLoS One. 2012;7:e31943.
Burton MJ, Mellor IR, Duce IR, Davies TGE, Field LM, Williamson MS. Differential resistance of insect sodium channels with kdr mutations to deltamethrin, permethrin and DDT. Insect Biochem Mol Biol. 2011;41:723–32.
Protopopoff N, Matowo J, Malima R, Kavishe R, Kaaya R, Wright A, et al. High level of resistance in the mosquito Anopheles gambiae to pyrethroid insecticides and reduced susceptibility to bendiocarb in north-western Tanzania. Malar J. 2013;12:149.
Ramphul U, Boase T, Bass C, Okedi LM, Donnelly MJ, Muller P. Insecticide resistance and its association with target-site mutations in natural populations of Anopheles gambiae from eastern Uganda. Trans R Soc Trop Med Hyg. 2009;103:1121–6.
Jones C, Liyanapathirana M, Agossa F, Weetman D, Ranson H, Donnelly M, et al. Footprints of positive selection associated with a novel mutation (N1575Y) in the voltage gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci USA. 2012;109:6614–9.
Mboera LEG, Magesa SM. The rise and fall of malarial sporozoite rates in Anopheles gambiae s.l. and An. funestus in north-eastern Tanzania, between 1934 and 1999. Ann Trop Med Parasitol. 2001;95:325–30.
Kristan M, Lines J, Nuwa A, Ntege C, Meek SR, Abeku TA. Exposure to deltamethrin affects development of Plasmodium falciparum inside wild pyrethroid resistant Anopheles gambiae s.s. mosquitoes in Uganda. Parasit Vectors. 2016;9:100.
Lynd A, Weetman D, Barbosa S, Yawson AE, Mitchell S, Pinto J, et al. Field, genetic and modelling approaches show strong positive selection acting upon an insecticide resistance mutation in Anopheles gambiae s.s. Mol Biol Evol. 2010;27:1117–25.
Alout H, Ndam NT, Sandeu MM, Djegbe I, Chandre F, Dabire RK, et al. Insecticide resistance alleles affect vector competence of Anopheles gambiae s.s. for Plasmodium falciparum field isolates. PLoS One. 2013;8:e63849.
Mitri C, Markianos K, Guelbeogo WM, Bischoff E, Gneme A, Eiglmeier K, et al. The kdr-bearing haplotype and susceptibility to Plasmodium falciparum in Anopheles gambiae: genetic correlation and functional testing. Malar J. 2015;14:391.
Authors’ contributions
BK was involved in the study design, supervised and participated in the implementation of field and laboratory work, organized and analysed data, drafted and revised the manuscript. PT was involved in field data collection. EJP and KS were involved in laboratory analysis of samples. WK, SM, FM and MJD were involved in the overall study design, helped to draft and revised the manuscript. MJD was also involved in data analysis, interpretation and revisions of the manuscript. All authors have read and approved the final manuscript.
Acknowledgements
The authors are grateful to the data collection team from NIMR Amani centre and to the Zeneti community. Thanks to Eric Lucas for critical comments. This work was supported by the malaria capacity development consortium (MCDC) through a PhD studentship scholarship to BK (MCDC is funded by The Wellcome Trust Grant Number WT084289MA). Additional funding support came from National Institute of Allergy and Infectious Diseases Grant AI082734 to MJD. The opinions expressed in this paper are those of the authors and may not reflect the position of their employing organizations nor their funding sources.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kabula, B., Tungu, P., Rippon, E.J. et al. A significant association between deltamethrin resistance, Plasmodium falciparum infection and the Vgsc-1014S resistance mutation in Anopheles gambiae highlights the epidemiological importance of resistance markers. Malar J 15, 289 (2016). https://doi.org/10.1186/s12936-016-1331-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-016-1331-5