Abstract
Physiochemical tissue inducers and mechanical stimulation are both efficient variables in cartilage tissue fabrication and regeneration. In the presence of biomolecules, decellularized extracellular matrix (ECM) may trigger and enhance stem cell proliferation and differentiation. Here, we investigated the controlled release of transforming growth factor beta (TGF-β1) as an active mediator of mesenchymal stromal cells (MSCs) in a biocompatible scaffold and mechanical stimulation for cartilage tissue engineering. ECM-derived hydrogel with TGF-β1-loaded alginate-based microspheres (MSs) was created to promote human MSC chondrogenic development. Ex vivo explants and a complicated multiaxial loading bioreactor replicated the physiological conditions. Hydrogels with/without MSs and TGF-β1 were highly cytocompatible. MSCs in ECM-derived hydrogel containing TGF-β1/MSs showed comparable chondrogenic gene expression levels as those hydrogels with TGF-β1 added in culture media or those without TGF-β1. However, constructs with TGF-β1 directly added within the hydrogel had inferior properties under unloaded conditions. The ECM-derived hydrogel group including TGF-β1/MSs under loading circumstances formed better cartilage matrix in an ex vivo osteochondral defect than control settings. This study demonstrates that controlled local delivery of TGF-β1 using MSs and mechanical loading is essential for neocartilage formation by MSCs and that further optimization is needed to prevent MSC differentiation towards hypertrophy.
Similar content being viewed by others
Introduction
Articular cartilage defects can be caused by traumatic injury, rheumatoid arthritis, or osteoarthritis, also called degenerative joint disease1,2. This tissue has a limited capacity for self-repair after damage, with consequences such as the loss of chondrocyte viability and function and, finally, permanent tissue degeneration3. Existing clinical treatments for cartilage defects, including autologous chondrocyte implantation (ACI), micro-fracture, mosaicplasty, and allograft implants, are not successful in fully restoring functional articular cartilage3,4. Hence, fibrous tissue, hypertrophic cartilage, or nonfunctional mechanical tissue are often the outcomes5,6.
Recently, decellularized ECM materials have become popular because the matrices retain the native structure of cartilage matrix, which provides cells with both mechanical and biochemical signals to promote stem cell fate and ultimately tissue regeneration7,8. The ECM materials can inherently induce stem cell differentiation and tissue regeneration, which may be an attractive alternative from both cost and regulatory standpoints9. On the other hand, MSCs do not have the potential for chondrogenic differentiation in the absence of chondrogenic stimuli, such as TGF-β or mechanical stimulation10,11. Members of the TGF-β superfamily have essential roles in cartilage differentiation through autocrine and paracrine signaling12,13. In this context, TGF-β1 is essential for cartilage-like tissue formation14,15 and several studies have confirmed its essential roles in cartilage tissue differentiation, regeneration, and healing16,17.
However, in addition to the chondrogenic stimuli, delivery methods have impressive effects on the final results18,19. On the other hand, the alginate-based systems are ideal candidates for bioactive molecule delivery because of their biocompatibility, flexibility in size and shape, and encapsulation efficiency20. Furthermore, alginate MSs allow for controlled diffusion rates of macromolecules20.
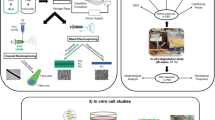
The objective of this study was to develop an alginate-based delivery vehicle for TGF-β1 and test its efficacy in inducing human MSC chondrogenesis and neocartilage formation, together with decellularized ECM-derived hydrogels in both in vitro and ex vivo microenvironments21,22. The decellularized ECM-derived hydrogel was combined with alginate MSs loaded with TGF-β1 (Fig. 1A) to promote its bioactivity retention in the fabricated hydrogel and stimulate the chondrogenesis of encapsulated MSCs (Fig. 1B). Furthermore, an osteochondral ex vivo explant defect model was used to assess the early cellular responses to complex multiaxial loading in a confined microenvironment via a bioreactor system (Fig. 1C).
Schematic illustrations of (A) production process of Alg–Ca microspheres (MSs) enclosing TGF-β1, and conjugated hydrogel formation through ionic crosslinking between Alg and Ca ions, (B) different ECM-derived hydrogels at physiological temperature and pH, and (C) production of ex vivo osteochondral plug filled with ECM-derived hydrogels loaded with TGF-β1/Alg–Ca/MSs and MSCs, followed by mechanical stimulation in a complex multiaxial bioreactor.
Materials and methods
Cartilage tissue decellularization
Articular cartilage was harvested from fresh bovine knees obtained from a local slaughterhouse. The femur cartilage was cut into thin, coin-like pieces with a diameter of 5 mm and a thickness of 2 mm. For decellularization, a combination of simultaneous physical and chemical treatments was utilized. For physical decellularization, samples were maintained at − 80 °C for three days, and then the tissue specimen was snap frozen and thawed in liquid nitrogen four times at 2-min intervals. The tissues were pulverized with mechanical force using a Molinex blender. After that, tissues were treated with 5% SDS for 8 h (Merck, Darmstadt, Germany) at 37 °C to achieve proper DNA extraction. Specimens were characterized histologically and by measuring the DNA content. Finally, samples were lyophilized.
Hydrogel synthesis
In order to synthesize the hydrogel, 1 g of the lyophilized cartilage was dissolved in 100 mL of 0.1 M acetic acid containing 5 mg/mL pepsin for 4 days at 4 °C. After that, the hydrogel was gelled by raising the pH to 7 at 37 °C within 5–7 min21,22. Scanning electron microscopy (SEM; AIS2100, Seron Technology, Korea) was used to observe the morphology of decellularized ECM and the hydrogel produced. For this, the specimens were dehydrated through a graded ethanol series (50–95% w/v), sputter-coated with gold (60 mA current, 25 kV, and a duration of 40 s), and examined using SEM.
Rheometric analyses
Viscoelastic properties were determined within the linear viscoelastic range of the gel. For this, the neutralized ECM-derived hydrogel was placed on a cylindrical module with dimensions of approximately 12 mm in diameter and 6 mm in thickness. Then, it was transferred to the parallel geometric flat probes, and the linear viscoelastic region was measured by performing a stress sweep at a frequency of 1 Hz. The elastic (storage modulus, Gʹ) and viscous (loss modulus, Gʹʹ) components of the sample were measured through a frequency sweep test at 0.01–100% strain with a constant strain detection of 5%.
Producing alginate-based MS containing TGF-β1
Spherical MSs of 200 µm diameter were generated using an electrostatic droplet generator. For this, 10 and 100 ng TGF-β1 was mixed in 1 mL sodium-alginate 1% (w/v) (viscosity 98 Pa.s.) in a calcium-free Krebs–Ringer HEPES buffer solution (CF-KRH, pH 7.4). The mixture was poured into a 5 mL plastic syringe and extruded from a 26-gauge stainless steel needle at 3 mL/h. Droplet formation was induced through an electrostatic encapsulation method and a high voltage rate set at 9 kV. The needle was connected to the cathode of a high-voltage DC generator and positioned above the gelation bath, which was connected to a ground wire. The distance between the tip of the needle and the surface of the gelation solution was 20 cm. The droplets were collected in a 100 mM CaCl2 solution, where the ionic reaction spontaneously began and crosslinking occurred among Alg molecules to form an ionic gel commonly called an egg-box-like structure. The Alg–Ca MSs were collected via centrifugation after 10 min of soaking in the gelation solution. The mean diameter of the MSs was determined based on the measurement of more than 100 droplets using an optical microscope.
TGF-β1 encapsulation efficiency
Alg–Ca MSs were dissolved in a sodium citrate (55 mM) solution just after preparation. The encapsulation efficiency of TGF-β1 in Alg–Ca MSs was determined by measuring TGF-β1 via an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Briefly, the solution containing dissolved TGF-β1 was added to pre-coated wells with anti-human TGF-β1 polyclonal antibodies. Then samples were treated with a biotin-conjugated mouse anti-TGF-β1 antibody and streptavidin–horseradish peroxidase, respectively. Color intensity was developed using a tetramethyl benzidine-hydrogen dioxide mixture and terminated with sulfuric acid 0.01 M. The absorbance of each well was determined using a spectrophotometer at 560 nm. We used 50 g MS in 0.5 ml hydrogel. All the procedures were repeated 3 times.
Release profile of TGF-β1
For this study, three experimental groups were analyzed (Table 1). The final concentration of TGF-β1 in each condition was 10 ng/mL. The prepared specimens were placed in 10 volumes of culture medium (α MEM; Gibco Technologies, Logan, UT) in a low-binding protein tube at 37 °C. The incubating medium was changed at the indicated time intervals. The amount of released TGF-β1 into the media was quantified by an ELISA kit (R&D Systems, Minneapolis, MN). Measurements were performed in triplicate, and the amount of protein release was expressed as a percentage of the initial amount of incorporated TGF-β1. The released TGF-β1 was quantified at the indicated time points over 21 days of incubation.
Characterization of MSCs
Human bone marrow samples were obtained from 9 patients who were 30–40 years old who underwent a total hip replacement at the Emam Khomeini Hospital following written informed consent. All methods were carried out in accordance with relevant guidelines and regulations. Also, all experimental protocols were approved by Tehran University of Medical Sciences with the licensing committee code number of IR.TUMS.VCR.REC.1396.3152. MSCs were isolated as described in our previous report23. Briefly, bone marrow aspirates were diluted with phosphate-buffered saline (PBS), layered over Ficoll solution (Sigma-Aldrich), and centrifuged at 800 g for 20 min to collect mononuclear cells from the gradient interface. Then, mononuclear cells were cultured in Dulbecco’s modified Eagle’s medium–high glucose (DMEM–HG, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin/streptomycin. MSCs were incubated at 5% CO2 and 37 °C until 80% confluence. MSC were used at 3–4th passages for further analyses. They were seeded in tissue culture dishes at 1.0 × 103 cells/cm2. Then, the culture medium was changed to osteogenic, chondrogenic, or adipogenic ones for 21 days, as described previously23.
MSCs culture in hydrogel
MSCs viability was investigated by encapsulating them within various decellularized hydrogel groups (Table 2). The ECM-derived hydrogels after neutralization were mixed with MSCs at 1 × 106 cells/mL. 500 µL of hydrogels were poured into a 24-well tissue culture plate and gelled by incubation at 37 °C for 30 min. Then, the cell-laden hydrogel constructs were exposed to culture medium (DMEM–HG) supplemented with 10% fetal calf serum (FCS; Gibco Technologies) and 1% penicillin/streptomycin and incubated at 5% CO2 and 37 °C. On days 1, 7, and 21, ECM-derived hydrogels were stained with Calcein AM/propidium iodide (PI) (live/dead).
Ex vivo osteochondral explant and mechanical loading
Defects were generated from bovine stifle joint explants using a sterilized 4 mm trephine drill (Brutsch–Ruegger, Urdorf, CH) to centrally remove a full-thickness circular cylindrical cartilage biopsy in dimensions of 4 mm in diameter and 3 mm in depth, as previously described (Figs. 1C, 2A)24,25,26. Afterward, the obtained explants were cultured in DMEM–HG containing 10% FCS, 1% penicillin/streptomycin at 37°C, and 5% CO2 for 24 h to ensure the sterility of the explants. Then, specimens were placed in well plates and coated with 1% (w/v) low-gelling agarose (SeaPlaque Agarose, Lonza, Rockland, USA) to prevent cellular outgrowth from the subchondral bone. Osteochondral defects were individually filled with one of the four MSC-laden ECM-derived hydrogels as described above (Fig. 2B).
The specimens were exposed to mechanical stimulation using a custom designed four-station bioreactor, installed in a 5% CO2 incubator at 37 °C. The bioreactor concept implies an orthogonally rotating ball, which is pressed onto the explant sample27. By oscillating ball rotations, shear motions are generated that reproduce the joint kinematics occurring in vivo. Dynamic compression of the ball onto the sample is applied with linear actuators, while step motors generate the simultaneous oscillation of the ball.
In the current study, the explants were placed into custom sample holders made from PEEK (Fig. 2B). A ceramic hip ball (32 mm in diameter) was pressed onto the osteochondral explants to reach a displacement of 10% of the cartilage height (from the center area) to fully contact the cell-laden hydrogel and the surrounding cartilage (Fig. 2C). After that, loading groups were exposed to axial compression in a sinusoidal manner between 0.4 and 0.8 mm, resulting in an actual strain amplitude of 10–20% of the cartilage explant height at a frequency of 0.5 Hz and simultaneous shear motion by ball oscillation at ± 25° and 0.5 Hz19.
The maximal applied mechanical loads corresponded to 15 N, or approximately 0.35 MPa. This type of dynamic axial compression with superimposed sliding motion could more precisely simulate joint articulation compared to axial compression alone24,28. The mechanical loading was applied for 1 h per day (23 h without ball contact; free swelling between loading cycles) during 21 consecutive days. The chondrogenic differentiation of loaded MSCs was compared with that of the same cell-laden hydrogel constructs in the absence of mechanical stimulation.
Chondrogenic induction of MSC-laden hydrogel
The expression of chondrogenic markers in the different ECM hydrogel groups was evaluated by total RNA isolation at day 21 of culture from the ex vivo osteochondral explant model with or without mechanical stimulation. The encapsulated cells were released from the ECM-derived hydrogels by collagenase degradation for 1 h at 37 °C. Then, the total RNA was utilized for the synthesis of the complementary DNA with the first-strand cDNA synthesis kit (PrimeScript™; TaKaRa, Tokyo, Japan). Real-time quantitative PCR was performed on a StepOne™ real-time PCR system (Applied Biosystems, Foster City, CA, USA) using SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA). The data were expressed by the 2−ΔΔCT method using StepOne software. Expression of marker genes was normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primer sequences used for the chondrogenic marker genes, including collagen type II (COLII), collagen type X (COLX), aggrecan (ACAN) and SOX9, are presented in Table 3.
Histological analysis
The osteochondral defect (including the different ECM hydrogels) or the ECM hydrogels cultured alone were fixed in 70% methanol for 24 h, and then they were incubated for decalcification in EDTA 10% for 15 days, followed by paraffin embedding. Histological section (7 μm thickness) were stained with toluidine blue to assess nuclei and hydrogel deposition in the defect region.
SEM preparation
Cell-laden and cell-free ECM-derived hydrogels were prepared for SEM analysis after 7 days of incubation in 4% glutaraldehyde. Then, the samples were dehydrated with ascending grades of ethanol: 30, 40, 60, 70, 80, 90, and 95%. Afterward, the samples were coated with a gold layer. Microphotographs were obtained by scanning electron microscopy using a VEGA, TESCAN apparatus (Czech Republic).
Biochemical analysis: s-GAG and DNA content
Cell-hydrogel constructs removed from osteochondral defect models in the loaded and unloaded groups were collected for biochemical analysis. Cell-hydrogel constructs were digested overnight in 0.5 mg/mL proteinase K at 56 °C (2.5 U/mg, chromozyme assay; Roche, Mannheim, Germany). DNA content was measured using the QUANT-iT Picogreen, DS assay kit (Molecular Probes, Life Technologies). The total amounts of sulfated glycosaminoglycan (s-GAG) were determined by the dimethyl-methylene blue (DMMB) dye-binding assay25,26.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS, USA). Three different samples within the same experiment were examined for checking the reproducibility. All data are expressed as mean ± SD. A one-way ANOVA with a Tukey post hoc test was used to determine differences among the four groups, and an independent T-test was used to determine differences among two groups (p < 0.05 was considered statistically significant).
Results
Hydrogel microstructure and cell morphology
The SEM images of the decellularized cartilage or ECM-derived hydrogel surfaces, as well as longitudinal cross-sections before and after cell encapsulating, are shown in Fig. 3A. The SEM photographs revealed preservation of the native structures of ECM and a uniform fibrillar structure of the ECM-derived hydrogel. Moreover, a porous three-dimensional microstructure with interconnected pores composed of oriented and dense intermeshed fibers is shown, indicating a potentially favorable microenvironment for cell attachment and growth 9. The encapsulated cells in decellularized cartilage and ECM-derived hydrogels attached and remained round in shape or with an elliptic morphology (Fig. 3A), which implies an inherent cell-friendly property of the hydrogels9.
(A) SEM images of the decellularized cartilage and decellularized hydrogels before and after cell seeding on days 0 and 7, respectively (B) H&E and toluidine blue staining of decellularized cartilage and MSCs encapsulated in ECM-derived hydrogel (day 0) (C) Rheology test of hydrogels was done at replicates (Rep.) of 1, 2, and 3 (n = 3) (D) Microphotographs and size distribution of Alg–Ca and TGF-β1/Alg–Ca MS droplets.
Besides, H&E staining also showed that cartilage tissues were successfully decellularized (Fig. 3B), and DNA content was obtained at 0.03 ± 0.005 ng/mg of cartilage tissue of dry weight, which indicates the necessity of chemical treatment with SDS at lower concentrations as well as physical treatment using Snap freezing and mechanical force for proper decellularization of tissue. Also, encapsulated MSCs within the ECM-derived hydrogel stained with toluidine blue at day 0 are shown in Fig. 3B. The storage modulus (Gʹ) and loss modulus (Gʹʹ) of the cylindrical hydrogel did not change until 1% strain, and ECM-derived hydrogels showed linear viscoelasticity properties (Fig. 3C). In general, the storage modulus was higher than the loss modulus, indicating the dominant elastic characteristics of these hydrogels. The maximum storage modulus reached 30 kPa at 37 °C for ECM-derived hydrogel (Fig. 3C).
Characterization of TGF-β1/Alg–Ca MS
We evaluated the size distribution and diameter of Alg–Ca MSs to assess the reproducibility and uniformity of MSs for TGF- β1 encapsulation. As expected, Alg–Ca MSs could be obtained using the electrostatic system (Fig. 3D), as previously reported29,30. A narrow size range and size consistency among the created MSs are shown by the quantification of MS diameter, which revealed average sizes of 206 ± 17 µm and 202 ± 8 µm for Alg–Ca and TGF-β1/Alg–Ca MSs, respectively (Fig. 3D). These results agreed with previous studies and proved that incorporation of TGF-β1 in the Alg–Ca solution did not hinder particle sphericity and size distribution due to the opposing charges between the molecules as well as ionic gelation29,30. The narrow size distribution of the MSs was confirmed by the coefficients of variance (defined as a standard deviation divided by the average diameter of microspheres) of less than 5.4% and 7.9% for Alg–Ca MS and TGF-β1/Alg–Ca MS, respectively. The diameter of Alg–Ca MS was relatively uniform, as indicated by the coefficient of variance for the size distribution under 8%.
Release of encapsulated TGF-β1
Modulation of bioactive molecule delivery is recognized as a crucial factor for the applicability of scaffolds in tissue engineering and regenerative medicine21,22. The bioactive release profiles of TGF-β1 from the TGF-β1/Alg–Ca MS and TGF-β1/Alg–Ca MS/ECM-derived hydrogels are presented in Fig. 4. The amount of TGF-β1 in the supernatant of the solution was then measured, and encapsulation efficacy was determined at 79% ± 4.1%. The high degree of efficacy could be due to the availability of positive charge amino groups in TGF-β1 which could interact via -OH and carbonyl groups of Alg, forming an amide hydrogen bond, and in following TGF-β1/Alg–Ca availability, proceeded with ionic interaction with CaCl2.
An increasing release rate of TGF-β1 of more than 31% was observed within the initial 12 h of encapsulation in TGF-β1/Alg–Ca MS (Fig. 4). Fast release continued at 42% at 2 days, and cumulative release of TGF-β1 continued up to 94% at 21 days. This could be the consequence of the dissociation of Alg–Ca bonds due to the substitution by sodium or potassium ions present in culture media30,31.
The TGF-β1 release from TGF-β1/Alg–Ca MS/ECM-derived hydrogel was initially very low, 7% at day 2, and then reached about 32% at day 9, which represents a lower release compared to 51% of TGF-β1/Alg–Ca MS and 77% of TGF-β1/ECM-derived hydrogel (Fig. 4). The release rate from TGF-β1/Alg–Ca MS/ECM-derived hydrogel continued steadily until 21 days, at 52%. The TGF-β1/ECM-derived hydrogel groups showed an intermediate release rate (Fig. 4). This result indicated that encapsulation of TGF-β1 in Alg–Ca MS/ECM or in ECM alone hydrogels could provide reliable systems for sustainable delivery, and using both structures resulted in the most controllable release approach for sustained release of TGF-β1 as a bioactive substrate required for cell and cartilage tissue development.
Cellular viability in 3D hydrogels
The viability of the MSCs within the ECM-derived hydrogels containing TGF-β1 and Alg–Ca MS was evaluated using the fluorescence microscope. The samples were stained with Calcein AM/ propidium iodide (PI) after up to 21 days of culture. Cells at day 1 after encapsulation were viable and spherical (Fig. 5). Cell viability was excellent up to 21 days of culture, and MSCs could elongate and spread (white arrows). There were no significant differences between the various hydrogels.
(A) Images showing cell viability of MSC in the different ECM-derived hydrogels stained with Calcein Am/propidium iodide (PI) (live/dead). Hydrogels were encapsulated until 21 days. Only a few dead cells could be observed within the hydrogel up to 7 days, while the diagrams reveal more details on various groups. (B) Live cell content in various groups on 7, 14, and 21 days (C) Death cell content in various groups on 7, 14, and 21 days.
Dimensions of osteochondral explants
The generation of the chondral defect model was validated by repeated measurements of the osteochondral explants, and the reproducibility of the produced chondral defect was similar to the previous reported parameters 26. Briefly, explants from calf stifle joints showed an average diameter and height of 7.65 ± 15 mm and 9.34 ± 12 mm, respectively (Fig. 6A,C). The diameter and depth of the chondral defects generated in the middle of the explants showed a diameter of 3.82 ± 0.13 mm and a depth of 2.72 ± 0.12 mm (Fig. 6B,D).
Size distribution of (A) osteochondral plug and (B) defect, which indicate good reproducibility of osteochondral plugs. (C) Representative image of an osteochondral explant production using the compact drill press. (D) The trephine is adjusted to create the desired depth of the circular groove, which was adjusted by the digital drilling press.
Chondrogenic gene expression for ex vivo osteochondral explants
Results for COLII, ACAN, SOX9, and COLX gene expression are shown in Fig. 7. The results are normalized to MSCs cultured in 2D at day 0. In non-loaded TGF-β1/ECM-derived hydrogel conditions, the level of COLII expression did not change in compared to ECM hydrogel and Al–Ca Ms/ECM-derived hydrogel groups. Meanwhile, the TGF-β1/Alg–Ca MS/ECM-derived hydrogel condition showed increased COLII expression compared to the other groups. Interestingly, in gels containing TGF-β1/ECM-derived hydrogel under mechanical loading experimental conditions, COLII expression was increased (Fig. 7A).
Fold change of (A) collagen type II (COL ӀӀ), (B) aggrecan (ACAN), (C) SOX9 and (D) Collagen type X (COL X) expression for encapsulated MSCs in hydrogels at day 21, compared with encapsulated cells in ECM hydrogel just after encapsulation. Hydrogels were implanted in created defect plug as an ex vivo model and underwent mechanical load until 21 days. Polystyrene (PS) tissue plate used as control. (Mean ± SD; n = 4; *: p < 0.05, **: p < 0.01, #: p > 0.05).
Regarding ACAN expression level, in non-loaded conditions, the presence of TGF-β1 did not show any significant changes among groups. On the contrary, with mechanical loading, ACAN expression levels were significantly increased in all hydrogels, although this boost was limited in the Al–Ca MS/ECM hydrogel group (Fig. 7B). For SOX9, the highest expression level was observed for TGF-β1/ECM-derived hydrogel under mechanical load (Fig. 7C). The COLX did not show changes in gene expression levels in all conditions, except for a clear increase in the TGF-β1/Alg–Ca MS/ECM-derived hydrogel group under mechanical load (Fig. 7D).
Cellular growth and GAG content in ex vivo experiments
The DNA content showed significant differences among loaded and unloaded groups in the TGF-β1 containing groups, with higher DNA amounts in the unloaded samples (Fig. 8A). There was a noticeable drop in the GAG content in the TGF-β1/ECM hydrogel group when mechanical load was applied (Fig. 8B). Interestingly, the maximum levels of GAG and GAG/DNA content were observed in TGF-β1/Alg–Ca MS/ECM-derived hydrogel under loading conditions (Fig. 8B,C).
Histological observations of the ex vivo condition
Histological observations of the ex vivo condition depicted that the integration of the developed implants and the defect area is acceptable (Fig. 9). Furthermore, the color intensity and volume of the staining appeared greater for hydrogels containing TGF-β1 in both loading and non-loading conditions (Fig. 9). Nevertheless, these observations could only be considered very preliminary due to the lack of any quantification process. Results showed that the loaded specimens demonstrated a higher cartilage formation compared to the unload specimens.
Discussions
Previously, researchers are believed that healing cartilage defects such as osteoarthritis and rheumatoid arthritis were challenging and difficult to achieve32,33. Also, previous studies showed that mechanical stimuli are vital for developing and maintaining articular cartilage25,28. Researchers demonstrated that various mechanical stimuli are associated with the cartilage microenvironment, including hydrostatic, tension, compression, and shear26,28. Bioreactors can mimic physiological loading conditions of the natural tissue microenvironment and have been used to investigate cartilaginous tissue formation in vitro25,28.
Recalling that hydrogels are proper a class of materials that could be used in various tissue engineering applications34,35; here, a decellularized cartilage hydrogel containing Alg–Ca MS loaded with TGF-β1 for prolonged control release of this factor was investigated for its chondrogenic differentiation potential of MSC in in vitro and ex vivo models. The utilized bioreactor provided mechanical stress utilizing a moving and rolling ceramic ball (Fig. 1). This type of bioreactor would mimic compressive and shear forces approximating the kinematic motion of an articulating joint to determine whether applied forces affected stem cells’ fate36.
Despite similar amounts of DNA among the different experimental groups, we observed a significant increase in GAG production for TGF-β1/Alg–Ca MS/ECM-derived hydrogel after multi-axial mechanical load compared to the other groups. In addition, the TGF-β1 encapsulated in the Alg–Ca MSs provided sustained release of TGF-β1 up to 21 days which showed control release of TGF-β1 was effective on the quality of cartilage preservation. This is indicating that Alg–Ca MS retention of TGF-β1 is beneficial to obtain a longer and beneficial period of this growth factor for cartilage tissue engineering. This is in line with previous work where continuous mechanical load and presence of TGF-β1 induced a significant increase in the expression of chondrogenic markers and GAG content32,37,38.
However, the presence of TGF-β1 is also associated with an increase of the hypertrophic marker, type X collagen. In this context, we speculated that this hypertrophic driving effect of TGF-β1, could be due to its prolonged released and its long-term stimulation on the surrounding cells in the TGF-β1/Alg–Ca MS/ECM-derived hydrogel. As shown in Fig. 7, all the other hydrogel compositions did not induce type X collagen, even in presence of TGF-β1. From this point of view, the TGF-β1/Alg–Ca MS/ECM hydrogel looks the most promising, with respect to increase the chondrogenic markers and GAG/DNA ratio, while keeping a limited induction of hypertrophy. Although, it is presently difficult to suggest a proper reason to this observation, except for the presence of an increase in calcium ions.
It should be noted that as this work was a study on the differentiation of MSCs to chondrocytes, investigating the hypertrophy in the differentiated cells was not the aim of this study, while some proper markers among four previously mentioned genes; COL II, ACAN, SOX 9, and COL X could be used for this analysis. Studies showed that a promoted cell differentiation to chondrocytes could be occurred, characterized by increased expressions of COL X, MMP-13 and Runx239. Meanwhile, researchers noted that SOX 9, aggrecan and COL II expressions were down-regulated39. Accordingly, COL X is associated with MMP-13, and are expressed simultaneously40.
Although mechanical loading on the TGF-β1/ECM hydrogel has increased the expression of COL II, ACAN, and SOX9 significantly, the DNA and GAG contents of this hydrogel has been decreased after mechanical loading. Recalling that GAG results are representative of protein expression, while Col II, ACAN, and SOX9 show gene expression, differences among them is justified. Moreover, as their expression were measured via two separated experiments, it is noticeable that the experiment conditions may be altered. The other effective factor in this reverse trend is the un-encapsulating the TGF-β1 in the hydrogel. Studies showed that prompt releasing of growth factors could alter the proteins expression, effecting their signaling pathways41. However, our targeted group, TGF-β1/Alg–Ca MS/ECM-derived hydrogel, shows the same trend in gene and protein expression, demonstrating the acceptable efficiency of encapsulating the TGF-β1 in Alg–Ca MSs, and sustain releasing of them into ECM-derived hydrogel, promoting the proliferation and differentiation of MSCs appropriately.
The viability staining showed the presence of alive encapsulated MSCs in a uniform and homogenous distribution within the different hydrogels. Because of the nature of our hydrogels, which contain decellularized ECM and some of them alginate, it was difficult to quantify the Toluidine blue staining observed in Fig. 9. Other quantification methods, like S35 labeling of the newly synthesized proteoglycans, will need to be applied in future works.
Conclusions
In this work, we investigated the controlled release of TGF-β1 as an active mediator of MSCs in a biocompatible scaffold and mechanical stimulation for cartilage tissue engineering. For this aim, ECM-derived hydrogel containing alginate-based MSs loaded with TGF-β1 was developed for the improvement of chondrogenic differentiation of human MSCs. Moreover, physiological conditions were simulated by using ex vivo explants and a complex multiaxial loading bioreactor. Our results recommended that in the presence of applied mechanical stimuli and prolonged delivery of TGF-β1, the chondrogenic genes were upregulated compared to unloaded osteochondral constructs, which could exert substantial effects in biomimetic cartilage tissue formation. Prolonged TGF-β1 retention using Alg–Ca MS remains beneficial to obtain a more extended period growth factor in cartilage tissue engineering, while mechanical loading which mimics native existing pressure and force on cartilage had most effective impact on the expression of chondrogenic factors. Therefore, it is expected that the simultaneous implication of controlled delivery of growth factors and periodic mechanical force could synergistically induce cartilage tissue engineering and regeneration.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ACAN:
-
Aggrecan
- ACI:
-
Autologous chondrocyte implantation
- COLII:
-
Collagen type II
- COLX:
-
Collagen type X
- DMMB:
-
Dimethyl-methylene blue
- DMEM–HG:
-
Dulbecco’s modified Eagle’s medium–high glucose
- ECM:
-
Extracellular matrix
- FBS:
-
Fetal bovine serum
- FCS:
-
Fetal calf serum
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- MSCs:
-
Mesenchymal stromal cells
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- MSs:
-
Microspheres
- PBS:
-
Phosphate-buffered saline
- PI:
-
Propidium iodide
- SEM:
-
Scanning electron microscopy
- s-GAG:
-
Sulfated glycosaminoglycan
- TGF-β1:
-
Transforming growth factor beta
References
Fusco, M., Skaper, S. D., Coaccioli, S., Varrassi, G. & Paladini, A. Degenerative joint diseases and neuroinflammation. Pain Pract. 17(4), 522–532 (2017).
Yousefi, A. M., Hoque, M. E., Prasad, R. G. & Uth, N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: a review. J. Biomed. Mater. Res. Part A 103(7), 2460–2481 (2015).
Azami, M. & Beheshtizadeh, N. Identification of regeneration-involved growth factors in cartilage engineering procedure promotes its reconstruction. Regen. Med. 16(8), 719–731 (2021).
Xu, Y. et al. Unraveling of advances in 3D-printed polymer-based bone scaffolds. Polymers 14(3), 566. https://doi.org/10.3390/polym14030566 (2022).
Solanki, K., Shanmugasundaram, S., Shetty, N. & Kim, S.-J. Articular cartilage repair and joint preservation: A review of the current status of biological approach. J. Clin. Orthop. Trauma 22, 101602 (2021).
Vaish, A. et al. Biological reconstruction of the joint: Concepts of articular cartilage regeneration and their scientific basis. J. Clin. Orthop. Trauma 24, 101718 (2022).
Kim, Y. S., Majid, M., Melchiorri, A. J. & Mikos, A. G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 4(1), 83–95 (2019).
Yuan, J. et al. Biomimetic peptide dynamic hydrogel inspired by humanized defensin nanonets as the wound-healing gel coating. Chem. Eng. J. 470, 144266 (2023).
Bordbar, S. et al. Production and evaluation of decellularized extracellular matrix hydrogel for cartilage regeneration derived from knee cartilage. J. Biomed. Mater. Res. Part A. 108(4), 938–946 (2020).
Szychlinska, M. A. et al. Mesenchymal stem cell-based cartilage regeneration approach and cell senescence: Can we manipulate cell aging and function?. Tissue Eng. Part B Rev. 23(6), 529–539 (2017).
Cao, J. et al. Influence of autologous dendritic cells on cytokine-induced killer cell proliferation, cell phenotype and antitumor activity in vitro. Oncol. Lett. 12(3), 2033–2037 (2016).
Tekari, A., Luginbuehl, R., Hofstetter, W. & Egli, R. J. Transforming growth factor beta signaling is essential for the autonomous formation of cartilage-like tissue by expanded chondrocytes. PLoS ONE 10(3), e0120857 (2015).
Yu, Y. et al. Targeting loop3 of sclerostin preserves its cardiovascular protective action and promotes bone formation. Nat. Commun. 13(1), 4241 (2022).
Huey, D. J., Hu, J. C. & Athanasiou, K. A. Unlike bone, cartilage regeneration remains elusive. Science 338(6109), 917–921 (2012).
Luo, G. et al. Itaconic acid induces angiogenesis and suppresses apoptosis via Nrf2/autophagy to prolong the survival of multi-territory perforator flaps. Heliyon 9(7), e17909 (2023).
Wang, Q. et al. Cartilage-specific deletion of Alk5 gene results in a progressive osteoarthritis-like phenotype in mice. Osteoarthr. Cartil. 25(11), 1868–1879 (2017).
van der Kraan, P. M. Differential role of transforming growth factor-beta in an osteoarthritic or a healthy joint. J. Bone Metab. 25(2), 65–72 (2018).
Venkatesan, J. K. et al. Impact of mechanical stimulation on the chondrogenic processes in human bone marrow aspirates modified to overexpress sox9 via rAAV vectors. J. Exp. Orthop. 4(1), 22 (2017).
Li, X. et al. Three-dimensional sulfated bacterial cellulose/gelatin composite scaffolds for culturing hepatocytes. Cyborg Bionic Syst. 4, 0021 (2023).
Ciofani, G., Raffa, V., Menciassi, A., Micera, S. & Dario, P. A drug delivery system based on alginate microspheres: Mass-transport test and in vitro validation. Biomed. Microdevices 9(3), 395–403 (2007).
DeFail, A. J., Chu, C. R., Izzo, N. & Marra, K. G. Controlled release of bioactive TGF-β1 from microspheres embedded within biodegradable hydrogels. Biomaterials 27(8), 1579–1585 (2006).
Kim, J. et al. TGF-β1 conjugated chitosan collagen hydrogels induce chondrogenic differentiation of human synovium-derived stem cells. J. Biol. Eng. 9(1), 1–11 (2015).
Safari, F. et al. Human umbilical cord-derived scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. A. 107(8), 1793–1802 (2019).
Grad, S. et al. Sliding motion modulates stiffness and friction coefficient at the surface of tissue engineered cartilage. Osteoarthr. Cartil. 20(4), 288–295 (2012).
Jeon, J. E., Schrobback, K., Hutmacher, D. W. & Klein, T. J. Dynamic compression improves biosynthesis of human zonal chondrocytes from osteoarthritis patients. Osteoarthr. Cartil. 20(8), 906–915 (2012).
van Schaik, T. J. et al. Development of an ex vivo murine osteochondral repair model. Cartilage https://doi.org/10.1177/1947603518809402 (2018).
Wimmer, M. A. et al. Tribology approach to the engineering and study of articular cartilage. Tissue Eng. 10(9–10), 1436–1445 (2004).
Vainieri, M., Wahl, D., Alini, M., van Osch, G. & Grad, S. Mechanically stimulated osteochondral organ culture for evaluation of biomaterials in cartilage repair studies. Acta Biomater. 81, 256–266 (2018).
Khanmohammadi, M., Sakai, S. & Taya, M. Characterization of encapsulated cells within hyaluronic acid and alginate microcapsules produced via horseradish peroxidase-catalyzed crosslinking. J. Biomater. Sci. Polym. Ed. 30(4), 295–307 (2019).
Khanmohammadi, M., Zolfagharzadeh, V., Bagher, Z., Soltani, H. & Ai, J. Cell encapsulation in core-shell microcapsules through coaxial electrospinning system and horseradish peroxidase-catalyzed crosslinking. Biomed. Phys. Eng. Express 6(1), 015022 (2020).
Bahrami, N. et al. The ability of 3D alginate/polyvinyl alcohol cross-linked hybrid hydrogel to differentiate periodontal ligament stem cells into osteoblasts. Arch. Neurosci. https://doi.org/10.5812/ans.85118 (2019).
Schätti, O. et al. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur. Cell Mater. 22(214–225), b97 (2011).
Chen, S. et al. lncRNA Xist regulates osteoblast differentiation by sponging miR-19a-3p in aging-induced osteoporosis. Aging Dis. 11(5), 1058–1068 (2020).
Shen, W. et al. A polymeric hydrogel to eliminate programmed death-ligand 1 for enhanced tumor radio-immunotherapy. ACS Nano 17(23), 23998–24011 (2023).
Khan, M. U. A. et al. Fundamental properties of smart hydrogels for tissue engineering applications: A review. Int. J. Biol. Macromol. 254, 127882 (2024).
Li, J. et al. The influence of the representation of collagen fibre organisation on the cartilage contact mechanics of the hip joint. J. Biomech. 49(9), 1679–1685 (2016).
Li, Z., Kupcsik, L., Yao, S. J., Alini, M. & Stoddart, M. J. Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-β pathway. J. Cell. Mol. Med. 14(6a), 1338–1346 (2010).
Terraciano, V. et al. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells 25(11), 2730–2738 (2007).
Xiao, D. et al. Notch signaling regulates MMP-13 expression via Runx2 in chondrocytes. Sci. Rep. 9(1), 15596 (2019).
Borzí, R. M. et al. Matrix metalloproteinase 13 loss associated with impaired extracellular matrix remodeling disrupts chondrocyte differentiation by concerted effects on multiple regulatory factors. Arthritis Rheum. 62(8), 2370–2381 (2010).
Itatani, Y., Kawada, K. & Sakai, Y. Transforming growth factor-β signaling pathway in colorectal cancer and its tumor microenvironment. Int. J. Mol. Sci. 20(23), 5822 (2019).
Acknowledgements
This Study has been funded and supported by Tehran University of medical sciences (TUMS), Grant Number 34173. Also, many thanks for the supports that received from AO Foundation and Royan Institute.
Funding
Tehran University of medical science, AO Foundation and Royan institute partially supported this study.
Author information
Authors and Affiliations
Contributions
All authors are contributed in the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bordbar, S., Li, Z., Lotfibakhshaiesh, N. et al. Cartilage tissue engineering using decellularized biomatrix hydrogel containing TGF-β-loaded alginate microspheres in mechanically loaded bioreactor. Sci Rep 14, 11991 (2024). https://doi.org/10.1038/s41598-024-62474-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62474-5
- Springer Nature Limited