Abstract
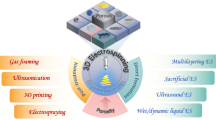
The goal of tissue engineering is to replace or regenerate damaged tissue. Scaffold fabrications and biomaterial selections are crucial factors for artificial tissue and bone tissue engineering, which are important due to the limited availability of tissue donors. This paper reviews the scaffold design considerations, manufacturing methods, and biomaterials for bone tissue engineering, and discusses current challenges and future perspectives. Scaffolds are required to have non-hazardous properties such as biocompatibility and biodegradability for the human body, and the necessary mechanical properties to support body weight, or to perform other roles, depending on the type of tissue. Moreover, scaffold structures such as porosity, pore size, and pore shape should be optimized to achieve cell viability and proliferation. Many conventional fabrication methods including thermally induced phase separation, emulsion freeze-drying, solvent casting, gas forming, and electrospinning have been studied and developed, but 3D printing is more suitable for bone tissue engineering because of its ability to manufacture complicated structures. Biomaterials can be divided into four categories: polymer, ceramic, metal, and composites. Composites blend two or more biomaterials to achieve desired properties for matching individual patient conditions. Finding a balance between fabrication method and biomaterial selection, in order to match properties between the scaffold and the target tissue, will be key to the field of bone tissue engineering in the future.


Similar content being viewed by others
Change history
30 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12541-023-00791-x
References
Viola, J., Lal, B., & Grad, O. (2003). The emergence of tissue engineering as a research field. National Science Foundation.
Langer, R., & Vacanti, J. P. (1993). Tissue engineering. Science, 260(5110), 920–926. https://doi.org/10.1126/science.8493529
Birla, R. (2014). Introduction to tissue engineering: Applications and challenges. Wiley.
Shafiee, A., & Atala, A. (2017). Tissue engineering: Toward a new era of medicine. Annual Review of Medicine, 68, 29–40. https://doi.org/10.1146/annurev-med-102715-092331
Lanza, R., Langer, R., Vacanti, J. P., & Atala, A. (2020). Principles of tissue engineering. Academic Press, an imprint of Elsevier.
Kim, B. S., Kwon, Y. W., Kong, J.-S., Park, G. T., Gao, G., Han, W., Kim, M.-B., Lee, H., Kim, J. H., & Cho, D.-W. (2018). 3d cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials, 168, 38–53.
Liu, Y., Zhou, G., & Cao, Y. (2017). Recent progress in cartilage tissue engineering-our experience and future directions. Engineering, 3(1), 28–35.
Zhai, Q., Dong, Z., Wang, W., Li, B., & Jin, Y. (2019). Dental stem cell and dental tissue regeneration. Frontiers of Medicine, 13(2), 152–159.
Liu, N., Ye, X., Yao, B., Zhao, M., Wu, P., Liu, G., Zhuang, D., Jiang, H., Chen, X., He, Y., et al. (2021). Advances in 3d bioprinting technology for cardiac tissue engineering and regeneration. Bioactive Materials, 6(5), 1388–1401.
Health Resources & Services Administration (HRSA): Organ Donation Statistics. Retrieved 01.09.2022. https://www.organdonor.gov/learn/organ-donation-statistics
Sodhi, H., & Panitch, A. (2021). Glycosaminoglycans in tissue engineering: A review. Biomolecules, 11(1), 29.
Biswal, T. (2021). Biopolymers for tissue engineering applications: A review. Materials Today: Proceedings, 41, 397–402.
Kim, B. W. (2018). Clinical regenerative medicine in urology. Springer.
Gopinathan, J., & Noh, I. (2018). Recent trends in bioinks for 3d printing. Biomaterials Research, 22(1), 1–15.
Wang, W., Zhang, B., Li, M., Li, J., Zhang, C., Han, Y., Wang, L., Wang, K., Zhou, C., Liu, L., et al. (2021). 3d printing of pla/n-ha composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Composites Part B: Engineering, 224, 109192.
Hussey, G. S., Dziki, J. L., & Badylak, S. F. (2018). Extracellular matrix-based materials for regenerative medicine. Nature Reviews Materials, 3(7), 159–173.
Williams, D. F. (2019). Challenges with the development of biomaterials for sustainable tissue engineering. Frontiers in bioengineering and biotechnology, 7, 127.
Laschke, M. W., & Menger, M. D. (2017). Life is 3d: Boosting spheroid function for tissue engineering. Trends in Biotechnology, 35(2), 133–144.
Duy Nguyen, B. T., Nguyen Thi, H. Y., Nguyen Thi, B. P., Kang, D.-K., & Kim, J. F. (2021). The roles of membrane technology in artificial organs: Current challenges and perspectives. Membranes, 11(4), 239.
Rao, S. H., Harini, B., Shadamarshan, R. P. K., Balagangadharan, K., & Selvamurugan, N. (2018). Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. International Journal o Biological Macromolecules, 110, 88–96.
Bala, Y., & Seeman, E. (2015). Bone’s material constituents and their contribution to bone strength in health, disease, and treatment. Calcified Tissue International, 97(3), 308–326.
Roddy, E., DeBaun, M. R., Daoud-Gray, A., Yang, Y. P., & Gardner, M. J. (2018). Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. European Journal of Orthopaedic Surgery & Traumatology, 28(3), 351–362.
Soundarya, S. P., Sanjay, V., Menon, A. H., Dhivya, S., & Selvamurugan, N. (2018). Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. International Journal of Biological Macromolecules, 110, 74–87.
Black, C. R., Goriainov, V., Gibbs, D., Kanczler, J., Tare, R. S., & Oreffo, R. O. (2015). Bone tissue engineering. Current Molecular Biology Reports, 1(3), 132–140.
Singh, D., Singh, D., & Han, S. S. (2016). 3d printing of scaffold for cells delivery: Advances in skin tissue engineering. Polymers, 8(1), 19.
Madrid, A. P. M., Vrech, S. M., Sanchez, M. A., & Rodriguez, A. P. (2019). Advances in additive manufacturing for bone tissue engineering scaffolds. Materials Science and Engineering: C, 100, 631–644.
Turnbull, G., Clarke, J., Picard, F., Riches, P., Jia, L., Han, F., Li, B., & Shu, W. (2018). 3d bioactive composite scaffolds for bone tissue engineering. Bioactive Materials, 3(3), 278–314.
Sarker, B., Li, W., Zheng, K., Detsch, R., & Boccaccini, A. R. (2016). Designing porous bone tissue engineering scaffolds with enhanced mechanical properties from composite hydrogels composed of modified alginate, gelatin, and bioactive glass. ACS Biomaterials Science & Engineering, 2(12), 2240–2254.
Jammalamadaka, U., & Tappa, K. (2018). Recent advances in biomaterials for 3d printing and tissue engineering. Journal of Functional Biomaterials, 9(1), 22.
Hussein, K. H., Park, K.-M., Kang, K.-S., & Woo, H.-M. (2016). Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Materials Science and Engineering: C, 67, 766–778.
Hou, Y., Wang, X., Yang, J., Zhu, R., Zhang, Z., & Li, Y. (2018). Development and biocompatibility evaluation of biodegradable bacterial cellulose as a novel peripheral nerve scaffold. Journal of Biomedical Materials Research Part A, 106(5), 1288–1298.
Gabbai-Armelin, P. R., Souza, M., Kido, H. W., Tim, C. R., Bossini, P. S., Fernandes, K. R., Magri, A. M. P., Parizotto, N. A., Fernandes, K. P. S., Mesquita-Ferrari, R. A., et al. (2017). Characterization and biocompatibility of a fibrous glassy scaffold. Journal of Tissue Engineering and Regenerative Medicine, 11(4), 1141–1151.
Asghari, F., Samiei, M., Adibkia, K., Akbarzadeh, A., & Davaran, S. (2017). Biodegradable and biocompatible polymers for tissue engineering application: a review. Artificial Cells, Nanomedicine, and Biotechnology, 45(2), 185–192.
Wheelton, A., Mace, J., Khan, S. W., & Anand, S. (2016). Biomaterials and fabrication to optimise scaffold properties for musculoskeletal tissue engineering. Current Stem Cell Research & Therapy, 11(7), 578–584.
Mao, D., Li, Q., Li, D., Chen, Y., Chen, X., & Xu, X. (2018). Fabrication of 3d porous poly (lactic acid)-based composite scaffolds with tunable biodegradation for bone tissue engineering. Materials & Design, 142, 1–10.
Prasadh, S., & Wong, R. C. W. (2018). Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Science International, 15(2), 48–55.
Qu, H., Fu, H., Han, Z., & Sun, Y. (2019). Biomaterials for bone tissue engineering scaffolds: A review. RSC Advances, 9(45), 26252–26262.
Mirzaali, M. J., Schwiedrzik, J. J., Thaiwichai, S., Best, J. P., Michler, J., Zysset, P. K., & Wolfram, U. (2016). Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone, 93, 196–211.
Qu, H., Zhang, W., Li, Z., Hou, L., Li, G., Fuh, J. Y., & Wu, W. (2022). Influence of thermal processing conditions on mechanical and material properties of 3d printed thin-structures using peek material. International Journal of Precision Engineering and Manufacturing, 23(6), 689–699.
Kim, M.-G., Kim, Y.-M., & Han, S.-Y. (2022). Mechanical reliability prediction of foldable displays using subcritical crack growth in siloxane-based cover window by two-point bending test. International Journal of Precision Engineering and Manufacturing, 23(11), 1301–1313.
Osterhoff, G., Morgan, E. F., Shefelbine, S. J., Karim, L., McNamara, L. M., & Augat, P. (2016). Bone mechanical properties and changes with osteoporosis. Injury, 47, 11–20.
Lee, J., Walker, J., Natarajan, S., & Yi, S. (2019). Prediction of geometric characteristics in polycaprolactone (pcl) scaffolds produced by extrusion-based additive manufacturing technique for tissue engineering. Rapid Prototyping Journal, 6, 66.
Murphy, C. M., Haugh, M. G., & O’brien, F. J. (2010). The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials, 31(3), 461–466.
Nagarajan, N., Dupret-Bories, A., Karabulut, E., Zorlutuna, P., & Vrana, N. E. (2018). Enabling personalized implant and controllable biosystem development through 3d printing. Biotechnology Advances, 36(2), 521–533.
Karageorgiou, V., & Kaplan, D. (2005). Porosity of 3d biomaterial scaffolds and osteogenesis. Biomaterials, 26(27), 5474–5491.
Akay, G., Birch, M., & Bokhari, M. (2004). Microcellular polyhipe polymer supports osteoblast growth and bone formation in vitro. Biomaterials, 25(18), 3991–4000.
Akin, F. A., Zreiqat, H., Jordan, S., Wijesundara, M. B., & Hanley, L. (2001). Preparation and analysis of macroporous tio2 films on ti surfaces for bone-tissue implants. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 57(4), 588–596.
Tarafder, S., Dernell, W. S., Bandyopadhyay, A., & Bose, S. (2015). Sro-and mgo-doped microwave sintered 3d printed tricalcium phosphate scaffolds: Mechanical properties and in vivo osteogenesis in a rabbit model. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 103(3), 679–690.
Wang, X., Xu, S., Zhou, S., Xu, W., Leary, M., Choong, P., Qian, M., Brandt, M., & Xie, Y. M. (2016). Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials, 83, 127–141.
Cavo, M., & Scaglione, S. (2016). Scaffold microstructure effects on functional and mechanical performance: Integration of theoretical and experimental approaches for bone tissue engineering applications. Materials Science and Engineering: C, 68, 872–879.
Grémare, A., Guduric, V., Bareille, R., Heroguez, V., Latour, S., & L’heureux, N., Fricain, J.-C., Catros, S., Le Nihouannen, D. (2018). Characterization of printed pla scaffolds for bone tissue engineering. Journal of Biomedical Materials Research Part A, 106(4), 887–894.
Chiu, Y.-C., Fang, H.-Y., Hsu, T.-T., Lin, C.-Y., & Shie, M.-Y. (2017). The characteristics of mineral trioxide aggregate/polycaprolactone 3-dimensional scaffold with osteogenesis properties for tissue regeneration. Journal of Endodontics, 43(6), 923–929.
Yang, F., Chen, C., Zhou, Q., Gong, Y., Li, R., Li, C., Klämpfl, F., Freund, S., Wu, X., Sun, Y., et al. (2017). Laser beam melting 3d printing of ti6al4v based porous structured dental implants: Fabrication, biocompatibility analysis and photoelastic study. Scientific Reports, 7(1), 1–12.
Pei, X., Ma, L., Zhang, B., Sun, J., Sun, Y., Fan, Y., Gou, Z., Zhou, C., & Zhang, X. (2017). Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication, 9(4), 045008.
Takahashi, Y., & Tabata, Y. (2004). Effect of the fiber diameter and porosity of non-woven pet fabrics on the osteogenic differentiation of mesenchymal stem cells. Journal of Biomaterials Science, Polymer Edition, 15(1), 41–57.
Chen, Z., Yan, X., Yin, S., Liu, L., Liu, X., Zhao, G., Ma, W., Qi, W., Ren, Z., Liao, H., et al. (2020). Influence of the pore size and porosity of selective laser melted ti6al4v eli porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Materials Science and Engineering: C, 106, 110289.
Gregor, A., Filová, E., Novák, M., Kronek, J., Chlup, H., Buzgo, M., Blahnová, V., Lukášová, V., Bartoš, M., Nečas, A., et al. (2017). Designing of pla scaffolds for bone tissue replacement fabricated by ordinary commercial 3d printer. Journal of Biological Engineering, 11(1), 1–21.
Sanzana, E. S., Navarro, M., Ginebra, M.-P., Planell, J. A., Ojeda, A. C., & Montecinos, H. A. (2014). Role of porosity and pore architecture in the in vivo bone regeneration capacity of biodegradable glass scaffolds. Journal of Biomedical Materials Research Part A, 102(6), 1767–1773.
Van Bael, S., Chai, Y. C., Truscello, S., Moesen, M., Kerckhofs, G., Van Oosterwyck, H., Kruth, J.-P., & Schrooten, J. (2012). The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted ti6al4v bone scaffolds. Acta Biomaterialia, 8(7), 2824–2834.
Montazerian, H., Zhianmanesh, M., Davoodi, E., Milani, A., & Hoorfar, M. (2017). Longitudinal and radial permeability analysis of additively manufactured porous scaffolds: Effect of pore shape and porosity. Materials & Design, 122, 146–156.
Jeong, C. G., & Hollister, S. J. (2010). Mechanical and biochemical assessments of three-dimensional poly (1, 8-octanediol-co-citrate) scaffold pore shape and permeability effects on in vitro chondrogenesis using primary chondrocytes. Tissue Engineering Part A, 16(12), 3759–3768.
Gong, B., Cui, S., Zhao, Y., Sun, Y., & Ding, Q. (2017). Strain-controlled fatigue behaviors of porous pla-based scaffolds by 3d-printing technology. Journal of Biomaterials Science, Polymer Edition, 28(18), 2196–2204.
Ferlin, K. M., Prendergast, M. E., Miller, M. L., Kaplan, D. S., & Fisher, J. P. (2016). Influence of 3d printed porous architecture on mesenchymal stem cell enrichment and differentiation. Acta Biomaterialia, 32, 161–169.
Wang, Y.-F., Barrera, C. M., Dauer, E. A., Gu, W., Andreopoulos, F., & Huang, C.-Y.C. (2017). Systematic characterization of porosity and mass transport and mechanical properties of porous polyurethane scaffolds. Journal of the Mechanical Behavior of Biomedical Materials, 65, 657–664.
Minas, C., Carnelli, D., Tervoort, E., & Studart, A. R. (2016). 3d printing of emulsions and foams into hierarchical porous ceramics. Advanced Materials, 28(45), 9993–9999.
Sampath, U. G., Ching, Y. C., Chuah, C. H., Sabariah, J. J., & Lin, P.-C. (2016). Fabrication of porous materials from natural/synthetic biopolymers and their composites. Materials, 9(12), 991.
Lee, J. S., Lee, H. K., Kim, J. Y., Hyon, S.-H., & Kim, S. C. (2003). Thermally induced phase separation in poly (lactic acid)/dialkyl phthalate systems. Journal of Applied Polymer Science, 88(9), 2224–2232.
Li, D., Krantz, W. B., Greenberg, A. R., & Sani, R. L. (2006). Membrane formation via thermally induced phase separation (tips): Model development and validation. Journal of Membrane Science, 279(1–2), 50–60.
Yang, H.-C., Wu, Q.-Y., Liang, H.-Q., Wan, L.-S., & Xu, Z.-K. (2013). Thermally induced phase separation of poly (vinylidene fluoride)/diluent systems: Optical microscope and infrared spectroscopy studies. Journal of Polymer Science Part B: Polymer Physics, 51(19), 1438–1447.
Kim, J. F., Kim, J. H., Lee, Y. M., & Drioli, E. (2016). Thermally induced phase separation and electrospinning methods for emerging membrane applications: A review. AIChE Journal, 62(2), 461–490.
Najjar, R., & Stubenrauch, C. (2009). Phase diagrams of microemulsions containing reducing agents and metal salts as bases for the synthesis of metallic nanoparticles. Journal of Colloid and Interface Science, 331(1), 214–220.
Whang, K., Thomas, C., Healy, K., & Nuber, G. (1995). A novel method to fabricate bioabsorbable scaffolds. Polymer, 36(4), 837–842.
Wu, X., Liu, Y., Li, X., Wen, P., Zhang, Y., Long, Y., Wang, X., Guo, Y., Xing, F., & Gao, J. (2010). Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomaterialia, 6(3), 1167–1177.
do Vale Morais, A.R., do Nascimento Alencar, É., Júnior, F.H.X., De Oliveira, C.M., Marcelino, H.R., Barratt, G., Fessi, H., Do Egito, E.S.T., & Elaissari, A. (2016). Freeze-drying of emulsified systems: A review. International Journal of Pharmaceutics, 503(1–2), 102–114.
Sola, A., Bertacchini, J., D’Avella, D., Anselmi, L., Maraldi, T., Marmiroli, S., & Messori, M. (2019). Development of solvent-casting particulate leaching (scpl) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Materials Science and Engineering: C, 96, 153–165.
Chia, O. C., Suhaimin, I. S., Kassim, S. A., Zubir, S. A., & Abdullah, T. K. (2019). Effect of modified solvent casting/particulate leaching (scpl) technique on the properties of bioactive glass reinforced polyurethane scaffold for biomedical applications. Journal of Physical Science, 30, 115–126.
Ghosal, K., Chandra, A., Roy, S., Agatemor, C., Thomas, S., Provaznik, I., et al. (2018). Electrospinning over solvent casting: Tuning of mechanical properties of membranes. Scientific Reports, 8(1), 1–9.
Cooper, A. I. (2000). Polymer synthesis and processing using supercritical carbon dioxide. Journal of Materials Chemistry, 10(2), 207–234.
Harris, L. D., Kim, B.-S., & Mooney, D. J. (1998). Open pore biodegradable matrices formed with gas foaming. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and the Australian Society for Biomaterials, 42(3), 396–402.
Jing, X., Mi, H.-Y., & Turng, L.-S. (2017). Comparison between pcl/hydroxyapatite (ha) and pcl/halloysite nanotube (hnt) composite scaffolds prepared by co-extrusion and gas foaming. Materials Science and Engineering: C, 72, 53–61.
Wu, Y. J., Chen, T., Chen, I.-F., Kuo, S. M., & Chuang, C. W. (2018). Developing highly porous collagen scaffolds by using alginate microsphere porogens for stem cell cultures. Materials Letters, 223, 120–123.
Kishan, A. P., & Cosgriff-Hernandez, E. M. (2017). Recent advancements in electrospinning design for tissue engineering applications: A review. Journal of Biomedical Materials Research Part A, 105(10), 2892–2905.
Jun, I., Han, H.-S., Edwards, J. R., & Jeon, H. (2018). Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. International Journal of Molecular Sciences, 19(3), 745.
Denchai, A., Tartarini, D., & Mele, E. (2018). Cellular response to surface morphology: Electrospinning and computational modeling. Frontiers in Bioengineering and Biotechnology, 155, 66.
Zhou, Y., Chyu, J., & Zumwalt, M. (2018). Recent progress of fabrication of cell scaffold by electrospinning technique for articular cartilage tissue engineering. International Journal of Biomaterials, 6, 66.
Soundarya, S. P., Menon, A. H., Chandran, S. V., & Selvamurugan, N. (2018). Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. International Journal of Biological Macromolecules, 119, 1228–1239.
Do, A.-V., Khorsand, B., Geary, S. M., & Salem, A. K. (2015). 3d printing of scaffolds for tissue regeneration applications. Advanced Healthcare Materials, 4(12), 1742–1762.
Hong, H., Eom, S., Lee, S. J., Youn, J., Kim, D., Chong, H. B., & Kim, D. S. (2022). Multi-scale fabrication techniques of collagen hydrogel for developing physiological 3d in vitro barrier model. International Journal of Precision Engineering and Manufacturing, 66, 1–28.
Yang, S., Leong, K.-F., Du, Z., & Chua, C.-K. (2002). The design of scaffolds for use in tissue engineering. Part ii. Rapid prototyping techniques. Tissue Engineering, 8(1), 1–11.
Jariwala, S. H., Lewis, G. S., Bushman, Z. J., Adair, J. H., & Donahue, H. J. (2015). 3d printing of personalized artificial bone scaffolds. 3D Printing and Additive Manufacturing, 2(2), 56–64.
Ma, P. X., Zhang, R., Xiao, G., & Franceschi, R. (2001). Engineering new bone tissue in vitro on highly porous poly (α-hydroxyl acids)/hydroxyapatite composite scaffolds. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials and The Japanese Society for Biomaterials, 54(2), 284–293.
Nie, L., Chen, D., Suo, J., Zou, P., Feng, S., Yang, Q., Yang, S., & Ye, S. (2012). Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze-drying method for bone tissue engineering applications. Colloids and Surfaces B: Biointerfaces, 100, 169–176.
Sola, A., Bertacchini, J., D’Avella, D., Anselmi, L., Maraldi, T., Marmiroli, S., & Messori, M. (2019). Development of solvent-casting particulate leaching (scpl) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Materials Science and Engineering: C, 96, 153–165.
Annabi, N., Mithieux, S. M., Weiss, A. S., & Dehghani, F. (2009). The fabrication of elastin-based hydrogels using high pressure CO2. Biomaterials, 30(1), 1–7.
Kim, S.-S., Park, M. S., Jeon, O., Choi, C. Y., & Kim, B.-S. (2006). Poly (lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials, 27(8), 1399–1409.
Mikos, A. G., Bao, Y., Cima, L. G., Ingber, D. E., Vacanti, J. P., & Langer, R. (1993). Preparation of poly (glycolic acid) bonded fiber structures for cell attachment and transplantation. Journal of Biomedical Materials Research, 27(2), 183–189.
Rahmati, M., Mills, D. K., Urbanska, A. M., Saeb, M. R., Venugopal, J. R., Ramakrishna, S., & Mozafari, M. (2021). Electrospinning for tissue engineering applications. Progress in Materials Science, 117, 100721.
Yoshimoto, H., Shin, Y., Terai, H., & Vacanti, J. (2003). A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials, 24(12), 2077–2082.
Ma, J. (2020). Environmentally sustainable management of 3d printing network: Decision support for 3d printing work allocation. International Journal of Precision Engineering and Manufacturing, 21(3), 537–544.
Guner, A., Bidare, P., Jiménez, A., Dimov, S., & Essa, K. (2022). Nozzle designs in powder-based direct laser deposition: A review. International Journal of Precision Engineering and Manufacturing, 66, 1–18.
Yao, G., Liu, P., Lu, S., & Yan, P. (2022). Design and analysis of additive manufactured flexure hinge with large stroke and high accuracy. International Journal of Precision Engineering and Manufacturing, 23(7), 753–761.
ISO/ASTM 52900. (2015). Additive manufacturing—general principles—terminology.
Wong, K. V., & Hernandez, A. (2012). A review of additive manufacturing. International Scholarly Research Notices, 6, 66.
Bose, S., Vahabzadeh, S., & Bandyopadhyay, A. (2013). Bone tissue engineering using 3d printing. Materials Today, 16(12), 496–504.
Szymczyk-Ziółkowska, P., Łabowska, M. B., Detyna, J., Michalak, I., & Gruber, P. (2020). A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybernetics and Biomedical Engineering, 40(2), 624–638.
Zhang, H., Moon, S. K., Ngo, T. H., Tou, J., Yusoff, Bin Mohamed, & M. A. (2020). Rapid process modeling of the aerosol jet printing based on gaussian process regression with latin hypercube sampling. International Journal of Precision Engineering and Manufacturing, 21(1), 127–136.
Zhang, L., Yang, G., Johnson, B. N., & Jia, X. (2019). Three-dimensional (3d) printed scaffold and material selection for bone repair. Acta Biomaterialia, 84, 16–33.
Cutolo, A., Neirinck, B., Lietaert, K., de Formanoir, C., & Van Hooreweder, B. (2018). Influence of layer thickness and post-process treatments on the fatigue properties of cocr scaffolds produced by laser powder bed fusion. Additive Manufacturing, 23, 498–504.
Luo, Y., Wu, C., Lode, A., & Gelinsky, M. (2012). Hierarchical mesoporous bioactive glass/alginate composite scaffolds fabricated by three-dimensional plotting for bone tissue engineering. Biofabrication, 5(1), 015005.
Shao, H., Ke, X., Liu, A., Sun, M., He, Y., Yang, X., Fu, J., Liu, Y., Zhang, L., Yang, G., et al. (2017). Bone regeneration in 3d printing bioactive ceramic scaffolds with improved tissue/material interface pore architecture in thin-wall bone defect. Biofabrication, 9(2), 025003.
Farkas, B., Dante, S., & Brandi, F. (2016). Photoinitiator-free 3d scaffolds fabricated by excimer laser photocuring. Nanotechnology, 28(3), 034001.
Wu, W., Geng, P., Li, G., Zhao, D., Zhang, H., & Zhao, J. (2015). Influence of layer thickness and raster angle on the mechanical properties of 3d-printed peek and a comparative mechanical study between peek and abs. Materials, 8(9), 5834–5846.
Yin, J., Yan, M., Wang, Y., Fu, J., & Suo, H. (2018). 3d bioprinting of low-concentration cell-laden gelatin methacrylate (gelma) bioinks with a two-step cross-linking strategy. ACS Applied Materials & Interfaces, 10(8), 6849–6857.
Ho, C. M. B., Mishra, A., Lin, P. T. P., Ng, S. H., Yeong, W. Y., Kim, Y.-J., & Yoon, Y.-J. (2017). 3d printed polycaprolactone carbon nanotube composite scaffolds for cardiac tissue engineering. Macromolecular Bioscience, 17(4), 1600250.
Darsell, J., Bose, S., Hosick, H. L., & Bandyopadhyay, A. (2003). From ct scan to ceramic bone graft. Journal of the American Ceramic Society, 86(7), 1076–1080.
Wang, C., Huang, W., Zhou, Y., He, L., He, Z., Chen, Z., He, X., Tian, S., Liao, J., Lu, B., et al. (2020). 3d printing of bone tissue engineering scaffolds. Bioactive Materials, 5(1), 82–91.
Aisenbrey, E. A., Tomaschke, A., Kleinjan, E., Muralidharan, A., Pascual-Garrido, C., McLeod, R. R., Ferguson, V. L., & Bryant, S. J. (2018). A stereolithography-based 3d printed hybrid scaffold for in situ cartilage defect repair. Macromolecular Bioscience, 18(2), 1700267.
Guillaume, O., Geven, M., Sprecher, C., Stadelmann, V., Grijpma, D., Tang, T., Qin, L., Lai, Y., Alini, M., De Bruijn, J., et al. (2017). Surface-enrichment with hydroxyapatite nanoparticles in stereolithography-fabricated composite polymer scaffolds promotes bone repair. Acta Biomaterialia, 54, 386–398.
Wang, Z., Abdulla, R., Parker, B., Samanipour, R., Ghosh, S., & Kim, K. (2015). A simple and high-resolution stereolithography-based 3d bioprinting system using visible light crosslinkable bioinks. Biofabrication, 7(4), 045009.
Chia, H. N., & Wu, B. M. (2015). Recent advances in 3d printing of biomaterials. Journal of Biological Engineering, 9(1), 1–14.
Elomaa, L., Keshi, E., Sauer, I. M., & Weinhart, M. (2020). Development of gelma/pcl and decm/pcl resins for 3d printing of acellular in vitro tissue scaffolds by stereolithography. Materials Science and Engineering: C, 112, 110958.
Lee, J. W., Ahn, G., Kim, D. S., & Cho, D.-W. (2009). Development of nano-and microscale composite 3d scaffolds using ppf/def-ha and micro-stereolithography. Microelectronic Engineering, 86(4–6), 1465–1467.
Sobral, J. M., Caridade, S. G., Sousa, R. A., Mano, J. F., & Reis, R. L. (2011). Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomaterialia, 7(3), 1009–1018.
Catros, S., Fricain, J.-C., Guillotin, B., Pippenger, B., Bareille, R., Remy, M., Lebraud, E., Desbat, B., Amédée, J., & Guillemot, F. (2011). Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication, 3(2), 025001.
Doraiswamy, A., Narayan, R., Harris, M., Qadri, S., Modi, R., & Chrisey, D. (2007). Laser microfabrication of hydroxyapatite-osteoblast-like cell composites. Journal of Biomedical Materials Research Part A, 80(3), 635–643.
Shahzad, K., Deckers, J., Zhang, Z., Kruth, J.-P., & Vleugels, J. (2014). Additive manufacturing of zirconia parts by indirect selective laser sintering. Journal of the European Ceramic Society, 34(1), 81–89.
Mazzoli, A. (2013). Selective laser sintering in biomedical engineering. Medical & Biological Engineering & Computing, 51(3), 245–256.
Bhatt, P. M., Kabir, A. M., Peralta, M., Bruck, H. A., & Gupta, S. K. (2019). A robotic cell for performing sheet lamination-based additive manufacturing. Additive Manufacturing, 27, 278–289.
Bae, E.-J., Kim, J.-H., Kim, W.-C., & Kim, H.-Y. (2014). Bond and fracture strength of metal–ceramic restorations formed by selective laser sintering. The Journal of Advanced Prosthodontics, 6(4), 266–271.
Liao, H.-T., Lee, M.-Y., Tsai, W.-W., Wang, H.-C., & Lu, W.-C. (2016). Osteogenesis of adipose-derived stem cells on polycaprolactone-\(\beta\)-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type i. Journal of Tissue Engineering and Regenerative Medicine, 10(10), 337–353.
Sun, H., He, S., Wu, P., Gao, C., Feng, P., Xiao, T., Deng, Y., & Shuai, C. (2016). A novel MgO–CaO–SiO\(_2\) system for fabricating bone scaffolds with improved overall performance. Materials, 9(4), 287.
Gu, B. K., Choi, D. J., Park, S. J., Kim, M. S., Kang, C. M., & Kim, C.-H. (2016). 3-dimensional bioprinting for tissue engineering applications. Biomaterials Research, 20(1), 1–8.
Bracaglia, L. G., Smith, B. T., Watson, E., Arumugasaamy, N., Mikos, A. G., & Fisher, J. P. (2017). 3d printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomaterialia, 56, 3–13.
Zhou, Z., Lennon, A., Buchanan, F., McCarthy, H. O., & Dunne, N. (2020). Binder jetting additive manufacturing of hydroxyapatite powders: Effects of adhesives on geometrical accuracy and green compressive strength. Additive Manufacturing, 36, 101645.
Yuan, L., Ding, S., & Wen, C. (2019). Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioactive Materials, 4, 56–70.
Sarvari, R., Keyhanvar, P., Agbolaghi, S., Roshangar, L., Bahremani, E., Keyhanvar, N., Haghdoost, M., Keshel, S. H., Taghikhani, A., Firouzi, N., et al. (2022). A comprehensive review on methods for promotion of mechanical features and biodegradation rate in amniotic membrane scaffolds. Journal of Materials Science: Materials in Medicine, 33(3), 1–26.
Chong, W., Kim, S., Yu, C., Woo, S., & Kim, K. (2021). Analysis of health management using physiological data based on continuous exercise. International Journal of Precision Engineering and Manufacturing, 22(5), 899–907.
Wang, Q.-D., & Guo, L.-X. (2021). Biomechanical role of nucleotomy in vibration characteristics of human spine. International Journal of Precision Engineering and Manufacturing, 22(7), 1323–1334.
Balagangadharan, K., Dhivya, S., & Selvamurugan, N. (2017). Chitosan based nanofibers in bone tissue engineering. International Journal of Biological Macromolecules, 104, 1372–1382.
LogithKumar, R., KeshavNarayan, A., Dhivya, S., Chawla, A., Saravanan, S., & Selvamurugan, N. (2016). A review of chitosan and its derivatives in bone tissue engineering. Carbohydrate Polymers, 151, 172–188.
Chen, L., Liu, J., Guan, M., Zhou, T., Duan, X., & Xiang, Z. (2020). Growth factor and its polymer scaffold-based delivery system for cartilage tissue engineering. International Journal of Nanomedicine, 15, 66. https://doi.org/10.2147/IJN.S249829
Subuki, I., Adnan, N., & Sharudin, R.W. (2018). Biodegradable scaffold of natural polymer and hydroxyapatite for bone tissue engineering: A short review. In AIP conference proceedings (vol. 2031, p. 020019). AIP Publishing LLC.
Dong, C., & Lv, Y. (2016). Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers, 8(2), 42.
Elango, J., Zhang, J., Bao, B., Palaniyandi, K., Wang, S., Wenhui, W., & Robinson, J. S. (2016). Rheological, biocompatibility and osteogenesis assessment of fish collagen scaffold for bone tissue engineering. International Journal of Biological Macromolecules, 91, 51–59.
Yang, B., Li, X., Shi, S., Kong, X., Guo, G., Huang, M., Luo, F., Wei, Y., Zhao, X., & Qian, Z. (2010). Preparation and characterization of a novel chitosan scaffold. Carbohydrate Polymers, 80(3), 860–865.
Xu, Y., Xia, D., Han, J., Yuan, S., Lin, H., & Zhao, C. (2017). Design and fabrication of porous chitosan scaffolds with tunable structures and mechanical properties. Carbohydrate Polymers, 177, 210–216.
Sheikh, F. A., Ju, H. W., Moon, B. M., Lee, O. J., Kim, J. H., Park, H. J., Kim, D. W., Kim, D. K., Jang, J. E., Khang, G., & Park, C. H. (2016). Hybrid scaffolds based on plga and silk for bone tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. https://doi.org/10.1002/term.1989
Choi, H. J., Zhu, M., Kim, D., Choi, J. H., Kim, C. M., Jeong, Y. H., & Kim, G. M. (2021). Sustained drug release from a microcontainer fabricated using a polydimethylsiloxane stencil. International Journal of Precision Engineering and Manufacturing, 22(11), 1873–1881.
Temple, J. P., Hutton, D. L., Hung, B. P., Huri, P. Y., Cook, C. A., Kondragunta, R., Jia, X., & Grayson, W. L. (2014). Engineering anatomically shaped vascularized bone grafts with hascs and 3d-printed pcl scaffolds. Journal of Biomedical Materials Research - Part A. https://doi.org/10.1002/jbm.a.35107
Singh, D., Babbar, A., Jain, V., Gupta, D., Saxena, S., & Dwibedi, V. (2019). Synthesis, characterization, and bioactivity investigation of biomimetic biodegradable pla scaffold fabricated by fused filament fabrication process. Journal of the Brazilian Society of Mechanical Sciences and Engineering, 41(3), 1–13.
Feng, P., Guo, X., Gao, C., Gao, D., Xiao, T., Shuai, X., Shuai, C., & Peng, S. (2015). Diopside modified porous polyglycolide scaffolds with improved properties. RSC Advances, 5(68), 54822–54829.
Backes, E. H., Harb, S. V., Beatrice, C. A. G., Shimomura, K. M. B., Passador, F. R., Costa, L. C., & Pessan, L. A. (2021). Polycaprolactone usage in additive manufacturing strategies for tissue engineering applications: A review. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 6, 66.
Santis, R. D., Russo, A., Gloria, A., D’Amora, U., Russo, T., Panseri, S., Sandri, M., Tampieri, A., Marcacci, M., Dediu, V. A., Wilde, C. J., & Ambrosio, L. (2015). Towards the design of 3d fiber-deposited poly(-caprolactone)/iron-doped hydroxyapatite nanocomposite magnetic scaffolds for bone regeneration. Journal of Biomedical Nanotechnology, 11, 1236–1246. https://doi.org/10.1166/jbn.2015.2065
Ma, H., Feng, C., Chang, J., & Wu, C. (2018). 3d-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomaterialia, 79, 37–59. https://doi.org/10.1016/j.actbio.2018.08.026
Cox, S. C., Thornby, J. A., Gibbons, G. J., Williams, M. A., & Mallick, K. K. (2015). 3d printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Materials Science and Engineering C. https://doi.org/10.1016/j.msec.2014.11.024
Mondal, S., Pal, U., & Dey, A. (2016). Natural origin hydroxyapatite scaffold as potential bone tissue engineering substitute. Ceramics International, 42(16), 18338–18346.
Canillas, M., Pena, P., Aza, A. H. D., & Rodríguez, M. A. (2017). Calcium phosphates for biomedical applications. Boletin de la Sociedad Espanola de Ceramica y Vidrio. https://doi.org/10.1016/j.bsecv.2017.05.001
Miranda, P., Pajares, A., Saiz, E., Tomsia, A. P., & Guiberteau, F. (2008). Mechanical properties of calcium phosphate scaffolds fabricated by robocasting. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 85(1), 218–227.
Shao, H., He, J., Lin, T., Zhang, Z., Zhang, Y., & Liu, S. (2019). 3d gel-printing of hydroxyapatite scaffold for bone tissue engineering. Ceramics International. https://doi.org/10.1016/j.ceramint.2018.09.300
Rahmanian, M., Seyfoori, A., Dehghan, M. M., Eini, L., Naghib, S. M., Gholami, H., et al. (2019). Multifunctional gelatin-tricalcium phosphate porous nanocomposite scaffolds for tissue engineering and local drug delivery: In vitro and in vivo studies. Journal of the Taiwan Institute of Chemical Engineers. https://doi.org/10.1016/j.jtice.2019.04.028.
Kim, B., Ventura, R., & Lee, B. T. (2018). Functionalization of porous bcp scaffold by generating cell-derived extracellular matrix from rat bone marrow stem cells culture for bone tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. https://doi.org/10.1002/term.2529
Khodaei, M., Valanezhad, A., Watanabe, I., & Yousefi, R. (2017). Surface and mechanical properties of modified porous titanium scaffold. Surface and Coatings Technology, 315, 61–66.
Zhang, X., Li, X.-W., Li, J.-G., & Sun, X.-D. (2014). Preparation and mechanical property of a novel 3d porous magnesium scaffold for bone tissue engineering. Materials Science and Engineering: C, 42, 362–367. https://doi.org/10.1016/j.msec.2014.05.044
Cheng, M.-Q., Wahafu, T., Jiang, G.-F., Liu, W., Qiao, Y.-Q., Peng, X.-C., Cheng, T., Zhang, X.-L., He, G., & Liu, X.-Y. (2016). A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Scientific Reports, 6(1), 1–14.
Witte, F., Hort, N., Vogt, C., Cohen, S., Kainer, K. U., Willumeit, R., & Feyerabend, F. (2008). Degradable biomaterials based on magnesium corrosion. Current Opinion in Solid State and Materials Science, 12(5–6), 63–72.
Witte, F., Fischer, J., Nellesen, J., & Beckmann, F. (2006). Microtomography of magnesium implants in bone and their degradation. In Developments in X-ray Tomography V (vol. 6318, p. 631806). International Society for Optics and Photonics.
Wenger, M. P., Bozec, L., Horton, M. A., & Mesquida, P. (2007). Mechanical properties of collagen fibrils. Biophysical Journal, 93(4), 1255–1263.
Roeder, B. A., Kokini, K., Sturgis, J. E., Robinson, J. P., & Voytik-Harbin, S. L. (2002). Tensile mechanical properties of three-dimensional type i collagen extracellular matrices with varied microstructure. Journal of Biomechanical Engineering, 124(2), 214–222.
Fan, H., Wang, L., Zhao, K., Li, N., Shi, Z., Ge, Z., & Jin, Z. (2010). Fabrication, mechanical properties, and biocompatibility of graphene-reinforced chitosan composites. Biomacromolecules, 11(9), 2345–2351.
Schuster, M., Turecek, C., Kaiser, B., Stampfl, J., Liska, R., & Varga, F. (2007). Evaluation of biocompatible photopolymers i: Photoreactivity and mechanical properties of reactive diluents. Journal of Macromolecular Science, Part A, 44(5), 547–557.
Cifuentes, S. C., Frutos, E., González-Carrasco, J. L., Muñoz, M., Multigner, M., Chao, J., Benavente, R., & Lieblich, M. (2012). Novel plla/magnesium composite for orthopedic applications: A proof of concept. Materials Letters, 74, 239–242.
Staiger, M. P., Pietak, A. M., Huadmai, J., & Dias, G. (2006). Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials, 27(9), 1728–1734.
Sankaranarayanan, S., Jayalakshmi, S., & Gupta, M. (2012). Effect of individual and combined addition of micro/nano-sized metallic elements on the microstructure and mechanical properties of pure mg. Materials & Design, 37, 274–284.
Shin, M., Yoshimoto, H., & Vacanti, J. P. (2004). In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Engineering. https://doi.org/10.1089/107632704322791673
Kong, J., Wei, B., Groth, T., Chen, Z., Li, L., He, D., Huang, R., Chu, J., & Zhao, M. (2018). Biomineralization improves mechanical and osteogenic properties of multilayer-modified plga porous scaffolds. Journal of Biomedical Materials Research - Part A. https://doi.org/10.1002/jbm.a.36487
Doyle, S. E., Henry, L., McGennisken, E., Onofrillo, C., Bella, C. D., Duchi, S., O’Connell, C. D., & Pirogova, E. (2021). Characterization of polycaprolactone nanohydroxyapatite composites with tunable degradability suitable for indirect printing. Polymers, 13(2), 295.
Germiniani, L. G., da Silva, L. C., Plivelic, T. S., & Gonçalves, M. C. (2019). Poly (\(\varepsilon\)-caprolactone)/cellulose nanocrystal nanocomposite mechanical reinforcement and morphology: the role of nanocrystal pre-dispersion. Journal of Materials Science, 54(1), 414–426.
Li, Y., Ceylan, M., Shrestha, B., Wang, H., Lu, Q. R., Asmatulu, R., & Yao, L. (2014). Nanofibers support oligodendrocyte precursor cell growth and function as a neuron-free model for myelination study. Biomacromolecules, 15(1), 319–326.
Aghdam, R. M., Najarian, S., Shakhesi, S., Khanlari, S., Shaabani, K., & Sharifi, S. (2012). Investigating the effect of pga on physical and mechanical properties of electrospun pcl/pga blend nanofibers. Journal of Applied Polymer Science, 124(1), 123–131.
Levengood, S. K. L., & Zhang, M. (2014). Chitosan-based scaffolds for bone tissue engineering. Journal of Materials Chemistry B, 2(21), 3161–3184.
Hospodiuk, M., Dey, M., Sosnoski, D., & Ozbolat, I. T. (2017). The bioink: A comprehensive review on bioprintable materials. Biotechnology Advances, 35(2), 217–239.
Collins, M. N., Ren, G., Young, K., Pina, S., Reis, R. L., & Oliveira, J. M. (2021). Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Advanced Functional Materials, 31(21), 2010609.
Vella, J. B., Trombetta, R. P., Hoffman, M. D., Inzana, J., Awad, H., & Benoit, D. S. W. (2018). Three dimensional printed calcium phosphate and poly(caprolactone) composites with improved mechanical properties and preserved microstructure. Journal of Biomedical Materials Research - Part A, 106, 663–672. https://doi.org/10.1002/jbm.a.36270
Kawai, T., Shanjani, Y., Fazeli, S., Behn, A. W., Okuzu, Y., Goodman, S. B., & Yang, Y. P. (2018). Customized, degradable, functionally graded scaffold for potential treatment of early stage osteonecrosis of the femoral head. Journal of Orthopaedic Research. https://doi.org/10.1002/jor.23673
Acknowledgements
This study has been conducted with the support of the Korea Institute of Industrial Technology as “Development of controllable mechanical and biological properties scaffold based on additive manufacturing (KITECH JE-22-0012).”
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper is an invited paper (Invited Review).
The original online version of this article was revised: In this article the authors have been affiliated erroneously.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jang, JW., Min, KE., Kim, C. et al. Review: Scaffold Characteristics, Fabrication Methods, and Biomaterials for the Bone Tissue Engineering. Int. J. Precis. Eng. Manuf. 24, 511–529 (2023). https://doi.org/10.1007/s12541-022-00755-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12541-022-00755-7