Abstract
Astaxanthin (ASX), as a natural carotenoid compound, exists in various types of seafood and microorganisms. It has several possible beneficial therapeutic effects for patients with polycystic ovary syndrome (PCOS). Patients with PCOS also suffer from endoplasmic reticulum (ER) stress. In the present work, it was hypothesized that ER stress could be improved by ASX in PCOS patients. Granulosa cells (GCs) were obtained from 58 PCOS patients. The patients were classified into ASX treatment (receiving 12 mg/day for 60 days) and placebo groups. The expression levels of ER stress pathway genes and proteins were explored using Western blotting and quantitative polymerase chain reaction. To assess oxidative stress markers, follicular fluid (FF) was gained from all patients. The Student’s t test was used to perform statistical analysis. After the intervention, ASX led to a considerable reduction in the expression levels of 78-kDa glucose-regulated protein (GRP78), CCAAT/enhancer-binding protein homologous protein (CHOP), and X-box-binding protein 1 compared to the placebo group, though the reduction in the messenger RNA (mRNA) expression level of activating transcription factor 6 was not statistically significant. However, ASX significantly increased the ATF4 expression level. GRP78 and CHOP protein levels represented a considerable decrease in the treatment group after the intervention. In addition, a statistically significant increase was found in the FF level of total antioxidant capacity in the treatment group. Based on clinical outcomes, no significant differences were found between the groups in terms of the oocyte number, fertilization rate, and fertility rate, but the ASX group had higher rates of high-quality oocytes, high-quality embryo, and oocyte maturity compared to the placebo group. Our findings demonstrated that ER stress in the GCs of PCOS patients could be modulated by ASX by changing the expression of genes and proteins included in the unfolding protein response.
Trial registration This study was retrospectively registered on the Iranian Registry of Clinical Trials website (www.irct.ir; IRCT-ID: IRCT20201029049183N, 2020-11-27).
Similar content being viewed by others
Introduction
Polycystic Ovary Syndrome (PCOS) is one of the most prevalent endocrine and metabolic dysfunctionsin women of reproductive age. It is the most common cause of anovulatory infertility, affecting 6–10% of these women1. PCOS is a heterogeneous disease that includes both endocrine and metabolic disorders. It has various symptoms, such as insulin resistance, hyperandrogenism, gonadotropin disorder, and anovulation dysfunction. The pathogenesis of PCOS is unknown; however, it is believed that this disease is linked to oxidative stress (OS), mitochondrial dysfunction, chronic low-grade inflammation, and metabolic disorders that impair normal ovarian function2,3,4,5. The endoplasmic reticulum (ER) is a critical organelle in all eukaryote cells that regulates the quality of secreted proteins6,7. Calcium homeostasis, protein folding, cell differentiation, lipid metabolism, and protein translocation are all controlled by ER homeostasis8,9. Nonetheless, the ER function capacity may exceed its normal limits under certain conditions, such as nutrient deprivation, acid–base instability, hypoxia, and reactive oxygen species (ROS) accumulation. These factors cause fluctuations that interfere with ER stability. Additionally, the accumulation of misfolded or unfolded proteins in ER causes ER stress induction10,11,12,13,14. ER stress is a critical local factor that interacts with OS and inflammation. The unfolding protein response (UPR) is activated in cells due to ER stress. UPR is a highly conserved process during evolution15. GRP78, a 78-kDa glucose-regulated protein, is a central factor in initiating UPR16. It activates 3ER transmembrane molecules, including RNA-dependent protein kinase (PKR)-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6), to initiate downstream UPR processes16. The apoptosis pathway is triggered by prolonged or severe ER stress17.
Cell death caused by ER stress is mediated by CCAAT/enhancer-binding protein homologous protein (CHOP)18. The primary function of IRE1 is to activate the UPR-related gene via X-box-binding protein 1 (XBP1)19. Furthermore, the principal roles of PERK are to reduce protein translation via eIF2 phosphorylation and to control transcription via ATF4 phosphorylation20. ATF6 activates the nucleus’ transcription factor, after moving from ER into the Golgi21. All three major UPR pathways have been found to regulate the pro-inflammatory transcriptional program via transcription factors, such as activator protein-1 and nuclear factor-κB(NF-κB)22. UPR and ER are essential in granulosa cells' physiological and pathological events (GCs)23.
UPR activation and ER stress play critical roles in the pathogenesis and progression of human diseases, particularly genetic disorders, autoimmune diseases, metabolic dysfunction, cancer, and neurodegenerative diseases24,25. Recent studies indicate that ER stress occurs in ovarian cells, influencing follicle formation, oocyte maturation, and ovulation26,27,28,29,30,31. Also ER stress has been linked to ovarian disease in a few studies. Excess androgen in GCs can activate the ER stress pathway, leading to apoptosis via death receptor 532. It is still difficult to develop an effective treatment for PCOS patients. In addition to different therapies for PCOS, lifestyle and diet changes have been suggested in this regard33. Diet and dietary factors play a major role in disease management. It has been shown that antioxidant interventions can improve PCOS complications, such as hormonal imbalances and metabolic disorders34. As a natural carotenoid compound, astaxanthin (ASX) is present in various kinds of seafood and microorganisms. Pluvialis-extracted ASX has been found to be safe and well-tolerated in daily dosages of 2–12 mg by the US Food and Drug Administration (FDA)35,36. Natural ASX has not been reported to be hazardous at any dose or for any duration of time in human investigations37. Various beneficial biological effects and activities are exhibited by ASX, including immunomodulatory and anti-inflammatory activity, protection against UV damage, cardioprotective effects, alleviation of metabolic syndrome, prevention of neuronal damage, anti-diabetic activity, inhibition of cell membrane peroxidation, and anti-aging and anti-cancer activities38,39. Additionally, previous studies have revealed the suppression of ER stress by ASX40,41. Thus, the present work aimed to determine the impacts of ASX supplementation on ER stress markers and OS in the GCs of infertile PCOS patients.
Methods
Trial design
Infertile PCOS patients aged 18–40 years were enrolled in this randomized clinical trial (RCT). They all met the Rotterdam criteria42 and were advised to undergo intracytoplasmic sperm injection (ICSI). PCOS was the only endocrinopathy in all of them and oligomenorrhea with oligo-ovulation due to the higher prevalence rate was the main reason for the inclusion of these patients.
Patients were excluded from the trial if they fulfilled any of the following criteria:
Severe endometriosis (stages 3 and 4 in terms of the revised AFS-rAFS classifying the endometriosis), FSH > 10 mg/mL, hyperprolactinemia, Cushing’s disease, ovarian tumors, thyroid disease, severe male factor infertility (particularly non-obstructive azoospermia), drug history affecting ovarian function in the 3 months prior to the study (steroids and oral contraceptive pills [OCPs]), female infertility factors other than cervical and tubal factors, any autoimmune disease, systemic disorders like metabolic syndrome, severe obesity and malnutrition (body mass index [BMI] over 35), hyperlipidemia, diabetes, and cardiovascular disease.
The participants were recruited from patients who were candidates for the ICSI protocol at Omid Clinic, Tehran, Iran, between November 2020 and September 2021. Although male factor indication for ICSI utilization appears to be constant, non-male factor indications remain controversial43. Some have advocated for the universal application of ICSI to all oocytes, regardless of the cause of infertility44,45. Moreover, several studieshave shown that conventional insemination of defective oocytes does not result in fertilization, whereas the use of ICSI increases fertilization and improve clinical outcome43,46. On the other hand, previous studies have shown that oocyte quality is low in patients with PCOS47,48. In our center, it has also been seen that PCOS patients with more dysmorphic oocytes have a lower fertilization rate after in vitro fertilization (IVF) compared to ICSI; accordingly, we employed ICSI, even though there was no male factor. The Ethics Committee of Tehran University of Medical Sciences approved the project (code:IR.TUMS.REC.1399.340) in accordance with relevant guidelines/regulations. The study was registered on the Iranian Registry of Clinical Trials (IRCT) website (IRCT-ID: IRCT20201029049183N1). Only a part of the results of the clinical trial registered with IRCT is presented in this paper. Before the intervention, each participant signed the informed consent form.
Intervention
ASX 12 mg/day (2 × 6 mg capsules; Astareal, Tokyo, Japan) was orally given to the participants in the treatment (ASX) group for 60 days. In the placebo group, the patients received 2 capsules containing edible paraffin every day for 8 weeks with the same appearance as ASX. The capsules were simultaneously produced by a similar company. According to a former study, the ASX dose was selected as 12 mg orally per day37. In addition to following their normal daily routine, patients had to notify the researchers of any changes to their activity level or diet. To monitor participants’ dietary intake or activity levels, a 3-day food diary was collected during the study from all participants (1 weekend day and 2 weekdays). The Nutritionist IV program was used to estimate dietary intake. Further, patients completed a validated form of the 7-item International Physical Activity Questionnaire (IPAQ) at the start and end of the trial. To ensure compliance during the trial, all participants received daily reminders to take their supplements and were also asked to return the empty supplement bottles.
Randomization
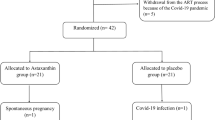
First, 58 women were included in the work. The participants were randomly assigned to control (placebo) and intervention (ASX) groups through blocked randomization. The block size was 4 and contained letters A and B (representing the intervention and control groups). Sequentially numbered sealed opaque envelopes were used to conceal the contents. To avoid bias, participants were kept apart from other researchers during randomization. Figure 1 illustrates the study map. In this study, the statistician, patients, and investigator were unaware of the grouping, and the decipherer was not part of the research team. The ASX and placebo capsules were randomly distributed to the trial participants in identical bottles containing 60 capsules. It should be noted that the medicinal content of each bottle was labeled in the form of a code by someone other than the research team, and the research team was unaware of its interpretation.
Ovarian stimulation protocol
An antagonistic regimen was administered to all patients (a flexible antagonist regime) for controlled ovarian stimulation. Prior to the ovarian stimulation cycle, all patients received OCPs (Ovocept LD, Abureihan, Iran) for 21 days. Briefly, 150–300 IU/day of recombinant follicle-stimulating hormone (Gonal-F, Merck Serono SA, Switzerland) was prescribed from the third day of the menstrual cycle. The optimum dosage was set considering the estradiol concentration and ovarian response. After monitoring the ovaries, when at least 2 follicles with the size of 14–15 mm were present, 0.25 mg/day cetrorelix acetate, Cetrotide (Merck Serono SA, Switzerland) was administered as the gonadotropin-releasing hormone antagonist. Cetrotide consumption was discontinued after reaching a diameter of 18 mm ≥ 2 for follicles; however, 10,000 IU human chorionic gonadotropin (hCG) was administered (Ovitrelle, Merck Serono SA, Switzerland), and oocytes were retrieved after 36 h through transvaginal ultrasound-guided aspiration49.
Sample preparation
Samples were prepared according to a previous study49. Follicles were aspirated without blood contamination and flushing on the oocyte retrieval day. All FFs were collected from each patient and centrifuged at 3000 g for 15 min. Furthermore, 5 mL of the supernatant was aliquoted and kept at − 80 °C for OS marker measurement. GCs were isolated by transferring the FF-derived pellet over 5 mL of Ficoll-Hypaque density gradient centrifugation (Lymphodex, Inno-Train, Germany). After centrifugation at 400 g for 20 min, the cells from the interface were gathered and rinsed at 600 g for 5 min. Moreover, 1 mL of phosphate-buffered saline (PBS; Sigma, Germany) with 1% bovine serum albumin (BSA; Sigma, Germany) was used for cell resuspension. Then, the cells were passed through a 40-μm cell strainer (BD Biosciences, CA, USA), and GCs were collected accordingly. Next, the cells were processed for the extraction of RNA and protein.
Follicular fluid analysis
Follicular fluid (FF) was assessed for superoxide dismutase (SOD), total antioxidant capacity (TAC), and malondialdehyde (MDA) using colorimetric assay kits (Navand Salamat, Tehran, Iran). All OS parameters were detected in duplicate.
RNA extraction and real-time polymerase chain reaction
Total RNA was manually extracted from GCs through a solution of RNX-PLUS (SinaClon, Tehran, Iran) based on the manufacturer’s protocol. The RNA concentration and purity were calculated via a spectrophotometer (Biochrom WPA Biowave, Cambridge, UK). Then, a complementary DNA (cDNA) synthesis kit (SinaClon, Tehran, Iran) was used to perform cDNA synthesis based on the manufacturer’s protocol. A RealQ Plus 2 × Master Mix Green (Amplicon, Denmark) was applied to define the gene expression levels. Additionally, polymerase chain reactions (PCRs) were conducted with specific primers for XBP1, ATF4, ATF6, GRP78, CHOP, and the glyceraldehyde‐3‐phosphate dehydrogenase as a housekeeping gene. All the reactions were performed twice by a Light Cycler 96 System (Roche, Germany). The messenger RNA (mRNA) expression of genes corresponding to the calibrator sample was calculated using the Livak method (2−ΔΔCt; Livak and Schmittgen, 2001). In the real-time PCR, 25 samples in each group were used for analysis. Table 1 presents the specific primer sequences.
Western blot analysis
As described earlier50, RIPA buffer was employed to lyse cells to extract total protein. After centrifugation at 14,000 rpm and 4 °C for 20 min, a BCA Protein Quantification kit was used to measure the protein concentration based on the manufacturer’s instructions. An equal volume of 2X Laemmli sample buffer was added to the cell lysates. Next, the lysates (20 μg) were exposed to SDS-PAGE, followed by boiling for 5 min and transferring them to a 0.2-μm immune-Blot polyvinylidene difluoride membrane (Cat No: 162–017,777; Bio-Rad Laboratories, CA, USA). Then, the membranes were blocked with 5% BSA (Cat No: A-7888; Sigma Aldrich, MO, USA) for 1 h in 0.1% Tween 20. Subsequently, the membranes were incubated with Anti-GRP78 (Cat No: ab21685, Abcam), anti-chop (Cat No: ab194533, Abcam), and anti-beta actin-loading control antibodies (Cat No: ab8227; Abcam) at room temperature for 1 h. After washing the membranes with TBST, incubation was performed with a secondary antibody, goat anti-rabbit IgG H&L (HRP; Cat No: ab6721; Abcam). Next, incubation with enhanced chemiluminescence was conducted for the membranes for 1–2 min. The densitometry of protein bands was calculated using Gel Analyzer version 2010a (NIH, USA). Thirty-three samples in the Western blot, 17 in the treatment group, and 16 in the placebo group were used for analysis.
Clinical data
Data on harvested oocytes, including the number of mature oocytes, the quality of the oocytes, and the percentage of high-quality oocytes, were obtained on the day of the puncture. It was determined that the high-quality oocyte rate was equal to the number of good-grade oocytes divided by the total number of retrieved oocytes × 100 for each participant. According to previous data51,52, 6 factors were used to assess the quality of individual oocytes, including oocyte morphology, range of possible oocyte sizes, ooplasm features, the structure of the perivitelline space (PVS), zona pellucida (ZP), and the morphology of the polar body. Oocyte morphology can be “worst” (characterized by an overall dark color and/or ovoid shape),“average” (characterized by a somewhat darker overall color and/or ovoid shape), or “best” (characterized by a normal color and shape). The range of possible oocyte sizes encompassed worst (abnormally tiny or huge, below 120 μ or larger than 160 μ), average (do not differ from best by more than 10 μ), and best (greater than 130 μ and less than 150 μ). The ooplasm feature was another intended factor (worst: extremely granular and/or vacuolated and/or demonstrating numerous inclusions; average: moderately granular and/or showing a small number of inclusions; best: showing no granularity or inclusions). The other factors were the structure of the PVS (worst: excessively large PVS, lack of PVS, or highly granular PVS; average: moderately enlarged/small/less granular PVS; best: best size PVS and the absence of granules in PVS) and ZP (worst: extremely thin or thick < 10 μ or > 20 μ; average:do not deviate from best by more than 2 μ; best: best zona > 2 μ and < 18 μ). The last factor was the morphology of the polar body, which can be the worst or multiple PBs (granular and/or either small or large PBs), average (fair but not excellent), and best (normal size and shape). The total oocyte score (TOS) was calculated by adding up the values assigned to each of the parameters; in addition, values of − 1, 0, and 1 represented the worst, average, and best, respectively. Oocytes may have a + 6 TOS and − 6 TOS at their highest and lowest levels, respectively. Oocytes were checked 16–18 h after ICSI to determine fertilization rates. Data on embryo development was gathered 2–3 days after ICSI, including number, quality, and high-quality embryo rate. In this study, the high-quality embryo rate was calculated as the number of high-quality embryos divided by the number of successful fertilizations × 10053 for each participant. The embryo quality is determined by its cell number (blastomeres), its fragmentation, and the presence of multiple nuclei, pits, and vacuoles54. In accordance with the ASEBIR (Association for the Study of Reproductive Biology) criteria, grade A and B cleavage embryos were classified as high quality based on these factors. The rates of chemical pregnancy (as determined by the hCG test) and clinical pregnancy (as determined by ultrasound to observe the gestational sac) were computed during the study.
Statistical analysis
The data were presented as means ± SDs. The Kolmogorov–Smirnov test was used to confirm the normal distribution of data. Statistical analyses were performed by the paired Student’s ttest or independent sample ttest and Fisher’s exact test using SPSS version 22 (SPSS Inc., Chicago, Ill, USA). P values less than 0.05 were considered statistically significant. To compare gene expression as the primary outcome in this trial and due to a paucity of comparative research in this field, the standardized effect size was used to determine the sample size. Considering the large standardized Cohen’s effect size (d = 0.8), a type I error of 5% (a = 0.05), and a type II error of 20% (b = 0.2; power = 80%), the sample size was obtained 26 in each group. Considering the 10% loss to follow up, the sample size was calculated to be 29 subjects in each group.
Ethics approval and consent to participate
The Ethics Committee of Tehran University of Medical Sciences approved the project (Ethics committee reference number: IR.TUMS.REC.1399.340) and all research was performed in accordance with relevant guidelines/regulations. Written consent was obtained from all participants.
Results
Generally, during the intervention stage, 5 subjects had to withdraw from the study, including 3 subjects in the placebo group (2 cases for personal reasons and 1 case due to pregnancy) and 2 cases in the ASX group (due to pregnancy). Finally, 53 patients participated in this study, including 27 and 26 cases in the intervention and placebo groups, respectively (Fig. 1). No adverse symptoms or effects with the ASX supplementation were reported by the participants during the trial, and they had good compliance with the intervention. No significant differences were also found in the duration of infertility, mean age, BMI, and hormonal profile between the treatment and control groups at the beginning of the study (Table 2). According to gene expression results, the expression levels of CHOP (P < 0.0001; Fig. 2a), GRP78 (P < 0.05; Fig. 2b), and XBP1 (P < 0.001; Fig. 2c) were significantly decreased in the treatment group than in the placebo group. Although in the treatment group, the mRNA expression level of ATF6 (P = 0.073; Fig. 2d) was reduced, this reduction was not significant statistically. However, the ATF4 expression level (P < 0.05; Fig. 2e) significantly increased compared to the placebo group. Moreover, the expression levels of CHOP and GRP78 protein significantly decreased after the intervention in the treatment group than in the control group (P < 0.0001; Fig. 3a and P < 0.001; Fig. 3b, respectively). Based on the results of the FF analysis (Table 3), a statistically significant increase was found in the TAC level in the treatment group (P < 0.05). Nonetheless, there was no considerable difference in the FF levels of MDA and SOD between the treatment and control groups (P > 0.05). According to Table 4, no statistically significant difference was detected in the number of retrieved oocytes, the number of embryos, and fertilization rates (P > 0.05), though the proportion of MII and high-quality oocytes rates were considerably higher in the study group than in the control group. Additionally, the embryo quality improved following ASX therapy. The results indicated that the rate of high-quality embryos was significantly higher in the study group than in the control group (P < 0.05). It was also found that the ASX group had a 44.44% chemical pregnancy success rate compared to the placebo group (34.61% success rate; (9/26, Fisher’s exact test; P = 0.577; Fig. 4). Additionally, the clinical pregnancy rate was 30.76% (8/26) in the placebo group and 37.03% (10/27) in the ASX group (Fisher’s exact test; P = 0.773; Fig. 4).
The fold changes levels of CHOP (a), GRP78 (b), XBP1 (c), ATF6 (d), and ATF4 (e) in GCs of placebo and treatment groups. Statistical significance (p < 0.05) was assessed by t-test. The results showed that fold changes levels of ATF4 was significantly increased in the intervention group (P < 0.05). After intervention, it was found that in the ASX group, the fold changes levels of CHOP, GRP78, and XBP1 were significantly decreased compared to the control group, while the reduction level of ATF6 was not significant between two groups (P > 0.05). P: placebo; T: treatment. Placebo: n = 25, Treatment: n = 25. Differences between groups; *p < 0.05, ***p < 0.001 and ****p < 0.0001.
The protein expression levels of CHOP and GRP78 in the GCs of placebo and treatment groups. Western blot analyzed of the protein expression of Grp78 and Chop normalized to β-actin. Following intervention, the protein expression of Grp78 and Chop significantly reduced in treatment group compared to control group. Statistical significance (p < 0.05) was assessed by t-test. P: placebo; T: treatment. Placebo: n = 16, Treatment: n = 17. Differences between groups; ***p < 0.001 ****p < 0.0001.
Comparison of clinical and chemical pregnancy rate between study groups. The chemical pregnancy rate was 44.44% (12/27) in the ASX group and 34.61% (9/26) in the placebo group (Fisher’s exact test; 1-sided P = 0.327, 2-sided P = 0.577). Moreover, the clinical pregnancy rate was 37.03% (10/27) in the ASX group and 30.76% (8/26) in the placebo group (Fisher’s exact test; 1-sided P = 0.424, 2-sided P = 0.773). Placebo: n = 26, Treatment: n = 27.
Discussion
Based on the findings of the present clinical trial on the effects of ASX on infertile patients with PCOS, the FF levels of MDA were reduced by ASX, while SOD and TAC levels represented an increase. Moreover, a 60-day course of ASX in the present work resulted in increased ATF4 mRNA expression levels. However, the expression levels of GRP78, CHOP, XBP1, and ATF6 mRNA were reduced compared to the placebo group. Our findings revealed that the protein levels of GRP78 and CHOP were also reduced after the pharmacological intervention. There is strong evidence that OS can result in ER stress55. ER stress was prevented by the administration of antioxidants, confirming the potential effects of OS on inducing ethanol-elicited ER stress40,56,57. In turn; OS is also induced by the accumulation of unfolded proteins in ER by various mechanisms. Mitochondrial oxidative phosphorylation is stimulated by the activated BiP, a key signal molecule in the ERstress pathway, to produce ROS as a by-product. Furthermore, the enzymes of the NADPH oxidase (NOX) family increase ROS production, particularly through Nox2 and Nox4 isoforms under ER stress58,59,60. The role of OS and ER stress in PCOS pathogenesis is highlighted by recent publications23,61. To determine whether ASX is effective in treating PCOS, the expression levels of mRNA and protein in the ER stress pathway were measured in our study. Our study focused on ER stress levels in the GCs of infertile PCOS patients who received ASX or not. Former investigations revealed an incremented expression of UPR genes such asATF4, ATF6, CHOP, and XBP-1 in GCs in PCOS patients23. According to our findings, the mRNA levels of GRP78, spliced XBP1, and CHOP were significantly decreased compared to the placebo group; however, the mRNA level of ATF4 was significantly increased in the ASX group. Various studies demonstrated that the activation of UPR and ER stress is associated with chronic diseases, including inflammation, diabetes, obesity, inflammatory bowel disease, neuromuscular inflammation disease, respiratory inflammation disease, PCOS, and arthritis16. Based on the results of studies on type 2 diabetes and insulin resistance, hyperglycemia has a close relationship with ER stress61. It was found that androgens can increase the GRP78 expression level62. Previous studies have confirmed the activation of ER stress by hyperandrogenism in PCOS32. ER stress includes some molecular cascades comprising some enzymes and transcription factors to restore homeostasis16. Based on a previous paper, GRP78 and UPR activator proteins (PERK, IRE1, and ATF6) increased in PCOS patients compared to healthy subjects23. Our results indicated that GRP78 mRNA and protein levels were significantly reduced by pharmacological intervention with ASX. A rise in the GRP78 expression in human diseases is an indicator of elevated protein misfolding conditions in ER, as well as a marker, to measure ER stress16. Treatment with ASX led to a reduction in the GRP78 expression level, indicating that ER stress conditions were reduced by interventions in PCOS patients, at least partially. The findings also showed a significant reduction in XBP1 mRNA expression after treatment with ASX in PCOS patients. Therefore, it is to be expected that ASX reduces XBP1 and thus decreases UPR target genes. It was concluded that the XBP1 expression level was reduced, while the ATF4 expression demonstrated a significant increase. According to former research, the expression of Nuclear factor E2-related factor 2(NRF2) is promoted by ATF4 by inducing genes included in antioxidant functions63. Our work revealed that ASX might have a role in NRF2 promotion by increasing ATF4. As a result of GRP78 activation, the ATF6 branch of the UPR travels to the Golgi, where it is processed by S1P and S2P proteases. Entering the activated cytosolic domain of ATF6 into the nucleus induces the expression of genes that augment the degradation and translation attenuation of misfolded proteins64. Hence, ATF6 appears to provide cell protection against ER stress. Nevertheless, some reports suggest that ATF6 may also have a pro-apoptotic function65. Moreover, CHOP and XBP1 are transactivated by ATF666. Our results suggest that ASX is associated with a decrease in UTR target genes by decreasing the expression of AFT6, though reduced AFT6 expression was statistically insignificant in our work. Under severe or chronic ER stress, apoptosis is started via CHOP, as well as other mechanisms16. A CHOP activity was found in the ovary physiological circumstances and PCOS66. In the current work, ASX reduced the CHOP mRNA and protein expression levels after the intervention. By the reduced expression levels of GRP78 and CHOP, the survival branches of UPR are induced by ASX in GCs in PCOS patients. Further, it was reported that by increasing ATF4, the PERK and CHOP expression levels increased as well67,68. Consistent with our results, in the study of Bhuvaneswari et al., the liver tissue was protected by ASX against higher fructose and fat diet-induced damage by reducing the levels of PERK and ATF659. However, Shen et al. concluded that the brain damage was attenuated by ASX in an experimental Parkinson’s disease model by reducing CHOP and GRP78 levels69. Wang et al. demonstrated that ethanol-induced cardiomyopathy was inhibited by ASX in mice by reducing the levels of ATF6, PERK, GRP78, ATF4, and CHOP41. Demir et al. demonstrated that the testicular tissue was protected by ASX against torsion/detorsion-induced injury through the antioxidant activity and suppression of endoplasmic stress. The levels of ATF6, GRP78, and CHOP markers were significantly reduced due to ASX treatment70. Numerous studies have shown that the activation of UPR and ER stress is implicated in chronic diseases, including inflammation, diabetes, obesity, inflammatory bowel disease, arthritis, neuromuscular inflammation disease, respiratory inflammation disease, and PCOS16. Investigations on type 2 diabetes and insulin resistance revealed a close relation between hyperglycemia and ER stress61. It was also found that androgens can increase the GRP78 expression level62. Higher levels of testosterone are produced in women with PCOS, along with other androgen hormones. By increasing these hormones in women with PCOS, multiple complications appear, including weight gain, acne, infertility, excessive growth of hair in the body or face, and absent or irregular menstrual periods61. A close relation was evidenced between signaling pathways for OS, inflammation, and ER stress71,72. It can be deduced that ER stress can be partially modulated by ASX antioxidant effects in PCOS patients. Although several studies have identified OS in patients with PCOS, the experimental data regarding OS markers are inconsistent73,74,75,76. These contradictory results could be due to small sample sizes and using different methods. It appears that antioxidant therapy might be beneficial to women with PCOS based on the OS status of these women. Our work confirmed that the FF level of TAC was increased by ASX supplementation for 60 days, while there was no considerable difference in the FF levels of MDA and SOD between the treatment and control groups. According to various animal trials, ASX can reduce OS by decreasing 8-hydroxy-2′-deoxyguanosine (8-OHdG)77 and MDA78, though it can increase antioxidant enzymes78,79. A limited number of clinical trials have also been performed in this regard. The results of a systematic review and meta-analysis on the antioxidant effect of ASX in humans showed that ASX might be operative in the reduction of OS as ASX may decrease the total quantity of the specific lipid peroxidation effectively (ISP and MDA) and improve plasma antioxidant capability (TAC) while increasing a definite antioxidant enzyme (SOD). However, the antioxidant effects of ASX on humans are unclear80. Reproductive potential is lowered in women with PCOS regardless of ovulatory state81 due to changes in oocytes82, embryo, and endometrial competence83, along with infertility-related co-morbidities, as well as a higher chance of pregnancy complications84. Virtually, every factor that is associated with PCOS also affects reproductive potential independent of PCOS. Meiotic abnormalities and poor oocyte quality are more common in women with PCOS due to the elevated OS that drives the excessive generation of ROS81.
Ultimately, this study discovered that administering ASX to PCOS patients before assisted reproductive technology (ART) cycles enhanced the proportion of MII and high-quality oocytes, as well as the rate of high-quality embryos. It seems that in our study, ASX might have been associated with the high-quality rate of oocyte and embryo by decreasing OS. Compared to the control group, the fertilization rate of the ASX group was not statistically greater (P = 0.17). In the present study, ASX had no significant effect on the fertility rate compared to the placebo. There was an increase in chemical pregnancy rates from 34.61% in the placebo group to 44.44% in the ASX group. There was also a rise in the rate of clinical pregnancy from 30.76% in the placebo group to 37.03% in the ASX group. Although the ASX group had a higher rate of chemical and clinical pregnancies, these changes were not statistically significant. The small sample size, ASX dosage, exposure length, and other factors may have contributed to the lack of statistical significance in our investigation.
One strength of our study is the coverage of all PCOS phenotypes, providing a considerably larger potential to derive generalizable results. Moreover, managing the nutritional intake and physical activity are beneficial aspects of our research. Nonetheless, this study had some limitations as well. Given that our sample size was relatively small, it may be difficult to detect small changes in response to ASX treatment. In addition, our study’s follow-up period was short; non-significant improvements in ART outcomes may be meaningful with longer follow-ups. The study was also limited by the lack of objective measurement of patient compliance; more precisely, the serum or plasma levels of ASX could not be measured in this study. However, these results may shed fresh light on the potential involvement of ASX in modulating several conditions in PCOS patients. Future studies are needed with larger sample sizes and various doses and durations to corroborate our findings. It is recommended that ASX concentrations in serum or plasma be measured as well.
It is concluded that the molecular pathways of ER stress can be modified by ASX as a natural supplement through antioxidant activity. Therefore, it can influence the expression of genes and proteins involved in the UPR in the GCs. Furthermore, OS markers may be modulated by ASX in the FF of patients. In addition, ASX may improve some of the ART outcomes for PCOS patients. Thus, ASX administration may be beneficial for PCOS patients. Eventually, the results of our study indicated that ER stress could be used as a potential therapeutic target in PCOS.
Data availability
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- ASX:
-
Astaxanthin
- PCOS:
-
Polycystic ovary syndrome
- ER:
-
Endoplasmic reticulum
- GCs:
-
Granulosa cells
- FF:
-
Follicular fluid
- GRP78:
-
Glucose regulated protein78
- CHOP:
-
C/EBP homologous protein
- ATF4:
-
Activating transcription factor4
- ATF6:
-
Activating transcription factor 4
- XBP1:
-
X-box binding protein 1
- GAPDH:
-
Glyceraldehyde-3-Phosphate dehydrogenase
- ROS:
-
Reactive oxygen species
- NRF2:
-
Nuclear factor E2-related factor 2
- OS:
-
Oxidative stress
- UPR:
-
Unfolding protein response
- RCT:
-
Randomized clinical trial
- ICSI:
-
Intracytoplasmic sperm injection
- IBD:
-
Inflammatory bowel disease
- 8-OHdG:
-
8-Hydroxy-2′-deoxyguanosine
- BMI:
-
Body mass index
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- Tes:
-
Testosterone
- AMH:
-
Anti-Müllerian hormone
- PRL:
-
Prolactin
- TAC:
-
Total antioxidant capacity
- SOD:
-
Superoxide dismutase
- MDA:
-
Malondialdehyde
- PVS:
-
Perivitelline space
- ZP:
-
Zona pellucid
- ART:
-
Assisted reproductive technology
References
Bozdag, G., Mumusoglu, S., Zengin, D., Karabulut, E. & Yildiz, B. O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 31(12), 2841–2855 (2016).
Qiao, J. & Feng, H. L. Extra-and intra-ovarian factors in polycystic ovary syndrome: Impact on oocyte maturation and embryo developmental competence. Hum. Reprod. Update 17(1), 17–33 (2011).
Insenser, M., Montes-Nieto, R., Murri, M. & Escobar-Morreale, H. F. Proteomic and metabolomic approaches to the study of polycystic ovary syndrome. Mol. Cell. Endocrinol. 370(1–2), 65–77 (2013).
Barrea, L. et al. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr. Res. Rev. 31(2), 291–301 (2018).
Jia, L. et al. Homocysteine impairs porcine oocyte quality via deregulation of one-carbon metabolism and hypermethylation of mitochondrial DNA. Biol. Reprod. 100(4), 907–916 (2019).
Araki, K. & Nagata, K. Protein folding and quality control in the ER. Cold Spring Harb. Perspect. Biol. 4(8), a015438 (2012).
Hetz, C., Chevet, E. & Harding, H. P. Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov. 12(9), 703–719 (2013).
Kaufman, R. J. et al. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 3(6), 411–421 (2002).
Martins, A. S., Alves, I., Helguero, L., Domingues, M. R. & Neves, B. M. The unfolded protein response in homeostasis and modulation of mammalian immune cells. Int. Rev. Immunol. 35(6), 457–476 (2016).
Yoshida, H. ER stress and diseases. FEBS J. 274(3), 630–658 (2007).
Whiteside, T. The tumor microenvironment and its role in promoting tumor growth. Oncogene 27(45), 5904–5912 (2008).
Kato, Y. et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 13(1), 1–8 (2013).
Chang, C.-H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162(6), 1229–1241 (2015).
Cubillos-Ruiz, J. R., Bettigole, S. E. & Glimcher, L. H. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell 168(4), 692–706 (2017).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8(7), 519–529 (2007).
Hasnain, S. Z., Lourie, R., Das, I., Chen, A. C. H. & McGuckin, M. A. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 90(3), 260–270 (2012).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403(6765), 98–103 (2000).
Wang, X.-Z. et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol. Cell. Biol. 16(8), 4273–4280 (1996).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107(7), 881–891 (2001).
Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397(6716), 271–274 (1999).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10(11), 3787–3799 (1999).
Garg, A. D. et al. ER stress-induced inflammation: Does it aid or impede disease progression?. Trends Mol. Med. 18(10), 589–598 (2012).
Takahashi, N. et al. Activation of endoplasmic reticulum stress in granulosa cells from patients with polycystic ovary syndrome contributes to ovarian fibrosis. Sci. Rep. 7(1), 1–13 (2017).
Oakes, S. A. & Papa, F. R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 10, 173–194 (2015).
Wang, M. & Kaufman, R. J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529(7586), 326–335 (2016).
Cree, L. M. et al. Maternal age and ovarian stimulation independently affect oocyte mtDNA copy number and cumulus cell gene expression in bovine clones. Hum. Reprod. 30(6), 1410–1420 (2015).
Lee, H.-M. et al. Cigarette smoke impaired maturation of ovarian follicles and normal growth of uterus inner wall of female wild-type and hypertensive rats. Reprod. Toxicol. 73, 232–240 (2017).
Wu, Y. et al. Diabetes induces abnormal ovarian function via triggering apoptosis of granulosa cells and suppressing ovarian angiogenesis. Int. J. Biol. Sci. 13(10), 1297 (2017).
Park, H. J. et al. Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. J. Pineal Res. 64(2), e12458 (2018).
Vašíčková, K., Moráň, L., Gurín, D. & Vaňhara, P. Alleviation of endoplasmic reticulum stress by tauroursodeoxycholic acid delays senescence of mouse ovarian surface epithelium. Cell Tissue Res. 374(3), 643–652 (2018).
Liu, J. et al. Cadmium induces ovarian granulosa cell damage by activating PERK-eIF2α-ATF4 through endoplasmic reticulum stress. Biol. Reprod. 100(1), 292–299 (2019).
Azhary, J. M. et al. Endoplasmic reticulum stress activated by androgen enhances apoptosis of granulosa cells via induction of death receptor 5 in PCOS. Endocrinology 160(1), 119–132 (2019).
Fabian, E. & Elmadfa, I. Influence of daily consumption of probiotic and conventional yoghurt on the plasma lipid profile in young healthy women. Ann. Nutr. Metab. 50(4), 387–393 (2006).
Gharaei, R. et al. Antioxidant supplementations ameliorate PCOS complications: A review of RCTs and insights into the underlying mechanisms. J. Assist. Reprod. Genet. 38(11), 2817–2831 (2021).
Capelli, B. & Cysewski, G. Natural Astaxanthin: King of the Carotenoids 4–19 (Cyanotech Corporation, 2007).
Fuji, L. Chemical Indusctry Co. New Dietary Ingredient Notification for Astaxanthin Extracted from Haematococcus Algae US. Food Drug Adm. 1, 1–6 (2004).
Brendler, T. & Williamson, E. M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 33(12), 3090–3111 (2019).
Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 16(4), 355–364 (2011).
Ambati, R. R., Phang, S.-M., Ravi, S. & Aswathanarayana, R. G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—a review. Mar. Drugs 12(1), 128–152 (2014).
Lin, X., Zhao, Y. & Li, S. Astaxanthin attenuates glutamate-induced apoptosis via inhibition of calcium influx and endoplasmic reticulum stress. Eur. J. Pharmacol. 806, 43–51 (2017).
Wang, W. et al. Astaxanthin attenuates alcoholic cardiomyopathy via inhibition of endoplasmic reticulum stress-mediated cardiac apoptosis. Toxicol. Appl. Pharmacol. 412, 115378 (2021).
Eshre, T. R. Group A-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 81(1), 19–25 (2004).
Haddad, M. et al. Thoughts on the popularity of ICSI. J. Assist. Reprod. Genet. 38(1), 101–123 (2021).
Bhattacharya, S. & Hamilton, M. Conventional insemination versus intracytoplasmic sperm injection. The Lancet. 358(9293), 1645–1646 (2001).
Abu-Hassan, D. & Al-Hasani, S. The use of ICSI for all cases of in-vitro conception. Hum. Reprod. (Oxford, England). 18(4), 893–894 (2003).
Setti, A. S. et al. Relationship between oocyte abnormal morphology and intracytoplasmic sperm injection outcomes: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 159(2), 364–370 (2011).
Lai, Q. et al. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front. Med. 12(5), 518–524 (2018).
Gong, Y. et al. Growth hormone alleviates oxidative stress and improves oocyte quality in Chinese women with polycystic ovary syndrome: A randomized controlled trial. Sci. Rep. 10(1), 1–10 (2020).
Qasemi, M. et al. Cell-free mtDNA level and its biomarker potency for ART outcome are different in follicular fluid of PCOS and non-PCOS women. Mitochondrion 59, 30–36 (2021).
Jabarpour, M. et al. Hyperbilirubinemia-induced pro-angiogenic activity of infantile endothelial progenitor cells. Microvasc. Res. 118, 49–56 (2018).
Lazzaroni-Tealdi, E. et al. Oocyte scoring enhances embryo-scoring in predicting pregnancy chances with IVF where it counts most. PLoS ONE 10(12), e0143632 (2015).
Rienzi, L., Balaban, B., Ebner, T. & Mandelbaum, J. The oocyte. Hum. Reprod. 27(suppl_1), i2–i21 (2012).
Hershko-Klement, A. et al. Embryo quality and implantation rates are not influenced by total motile count values in an ICSI programme: A novel point of view. Int. J. Mol. Epidemiol. Genet. 3(3), 205 (2012).
Medicine, A. S. I. R. Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Reprod. Biomed. Online 22(6), 632–646 (2011).
Victor, P., Sarada, D. & Ramkumar, K. M. Crosstalk between endoplasmic reticulum stress and oxidative stress: Focus on protein disulfide isomerase and endoplasmic reticulum oxidase 1. Eur. J. Pharmacol. 892, 173749 (2021).
Cai, L. Alcoholic cardiomyopathy: Acetaldehyde, insulin insensitization and ER stress. J. Mol. Cell. Cardiol. 44(6), 979–982 (2008).
Lee, H. et al. Tyrosol, an olive oil polyphenol, inhibits ER stress-induced apoptosis in pancreatic β-cell through JNK signaling. Biochem. Biophys. Res. Commun. 469(3), 748–752 (2016).
Malhotra, J. D. & Kaufman, R. J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword?. Antioxid. Redox Signal. 9(12), 2277–2294 (2007).
Bhuvaneswari, S., Yogalakshmi, B., Sreeja, S. & Anuradha, C. V. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-κB-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress Chaperones 19(2), 183–191 (2014).
Santos, C. X. et al. Endoplasmic reticulum stress and Nox-mediated reactive oxygen species signaling in the peripheral vasculature: Potential role in hypertension. Antioxid. Redox Signal. 20(1), 121–134 (2014).
Bañuls, C. et al. Metabolic syndrome enhances endoplasmic reticulum, oxidative stress and leukocyte–endothelium interactions in PCOS. Metabolism 71, 153–162 (2017).
Segawa, T. et al. Androgen-induced expression of endoplasmic reticulum (ER) stress response genes in prostate cancer cells. Oncogene 21(57), 8749–8758 (2002).
Sarcinelli, C. et al. ATF4-dependent NRF2 transcriptional regulation promotes antioxidant protection during endoplasmic reticulum stress. Cancers 12(3), 569 (2020).
Hotamisligil, G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140(6), 900–917 (2010).
Lin, J. H., Walter, P. & Yen, T. B. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 3, 399–425 (2008).
Huang, N., Yu, Y. & Qiao, J. Dual role for the unfolded protein response in the ovary: Adaption and apoptosis. Protein Cell 8(1), 14–24 (2017).
Rozpedek, W. et al. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 16(6), 533–544 (2016).
Yao, Y. et al. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat. Commun. 8(1), 1–15 (2017).
Shen, D.-F. et al. Astaxanthin suppresses endoplasmic reticulum stress and protects against neuron damage in Parkinson’s disease by regulating miR-7/SNCA axis. Neurosci. Res. 165, 51–60 (2021).
Demir, S. et al. Astaxanthin protects testicular tissue against torsion/detorsion-induced injury via suppressing endoplasmic reticulum stress in rats. J. Investig. Surg. 35, 1–6 (2021).
Özcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306(5695), 457–461 (2004).
Higa, A. & Chevet, E. Redox signaling loops in the unfolded protein response. Cell. Signal. 24(8), 1548–1555 (2012).
Murri, M., Luque-Ramírez, M., Insenser, M., Ojeda-Ojeda, M. & Escobar-Morreale, H. F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Update 19(3), 268–288 (2013).
Wang, H., Ruan, X., Li, Y., Cheng, J. & Mueck, A. O. Oxidative stress indicators in Chinese women with PCOS and correlation with features of metabolic syndrome and dependency on lipid patterns. Arch. Gynecol. Obstet. 300(5), 1413–1421 (2019).
Oyawoye, O. A. et al. The interaction between follicular fluid total antioxidant capacity, infertility and early reproductive outcomes during in vitro fertilization. Redox Rep. 14(5), 205–213 (2009).
Turan, V., Sezer, E. D., Zeybek, B. & Sendag, F. Infertility and the presence of insulin resistance are associated with increased oxidative stress in young, non-obese Turkish women with polycystic ovary syndrome. J. Pediatr. Adolesc. Gynecol. 28(2), 119–123 (2015).
Kochi, T. et al. Inhibitory effects of astaxanthin on azoxymethane-induced colonic preneoplastic lesions in C57/BL/KsJ-db/db mice. BMC Gastroenterol. 14(1), 1–10 (2014).
Guo, S.-X. et al. Astaxanthin attenuates early acute kidney injury following severe burns in rats by ameliorating oxidative stress and mitochondrial-related apoptosis. Mar. Drugs 13(4), 2105–2123 (2015).
Fassett, R. G. & Coombes, J. S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 9(3), 447–465 (2011).
Wu, D., Xu, H., Chen, J. & Zhang, L. Effects of astaxanthin supplementation on oxidative stress. Int. J. Vitamin Nutr. Res. (2019).
Palomba, S. Is fertility reduced in ovulatory women with polycystic ovary syndrome? An opinion paper. Hum. Reprod. 36(9), 2421–2428 (2021).
Palomba, S., Daolio, J. & La Sala, G. B. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol. Metab. 28(3), 186–198 (2017).
Palomba, S., Piltonen, T. T. & Giudice, L. C. Endometrial function in women with polycystic ovary syndrome: A comprehensive review. Hum. Reprod. Update 27(3), 584–618 (2021).
Palomba, S. et al. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update 21(5), 575–592 (2015).
Acknowledgements
The authors wish to thank the participants and staff of the Omid fertility clinic who assisted with sample collection, as well as to Astareal and the Nanospide Pharmed Company for their support.
Funding
This study was financially supported by the Tehran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
F.A. and M.J.: designed and performed the experiments, provided the samples, statistical analysis, and prepared the manuscript; A.A.: assisted with study design, performed the study protocol and revised the manuscript; M.S.: assisted in performing the study protocol and revised the manuscript. S.L.: Data collection and data analysis. The final version was approved by all authors for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jabarpour, M., Aleyasin, A., Nashtaei, M.S. et al. Astaxanthin treatment ameliorates ER stress in polycystic ovary syndrome patients: a randomized clinical trial. Sci Rep 13, 3376 (2023). https://doi.org/10.1038/s41598-023-28956-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28956-8
- Springer Nature Limited
This article is cited by
-
Resveratrol ameliorates mitochondrial biogenesis and reproductive outcomes in women with polycystic ovary syndrome undergoing assisted reproduction: a randomized, triple-blind, placebo-controlled clinical trial
Journal of Ovarian Research (2024)
-
Astaxanthin treatment decreases pro‐inflammatory cytokines and improves reproductive outcomes in patients with polycystic ovary syndrome undergoing assisted reproductive technology: A randomized clinical trial
Inflammopharmacology (2024)