Abstract
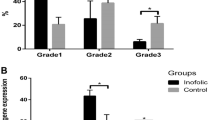
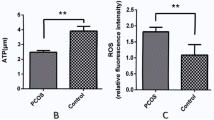
The increased levels of intracellular reactive oxygen species (ROS) in granulosa cells (GCs) may affect the pregnancy results in women with polycystic ovary syndrome (PCOS). In this study, we compared the in vitro fertilization and embryo transfer (IVF-ET) results of 22 patients with PCOS and 25 patients with tubal factor infertility and detected the ROS levels in the GCs of these two groups. Results showed that the PCOS group had significantly larger follicles on the administration day for human chorionic gonadotropin than the tubal factor group (P < 0.05); however, the number of retrieved oocytes was not significantly different between the two groups (P > 0.05). PCOS group had slightly lower fertilization, cleavage, grade I/II embryo, clinical pregnancy, and implantation rates and higher miscarriage rate than the tubal factor group (P > 0.05). We further found a significantly higher ROS level of GCs in the PCOS group than in the tubal factor group (P < 0.05). The increased ROS levels in GCs caused GC apoptosis, whereas NADPH oxidase 2 (NOX2) specific inhibitors (diphenyleneiodonium and apocynin) significantly reduced the ROS production in the PCOS group. In conclusion, the increased ROS expression levels in PCOS GCs greatly induced cell apoptosis, which further affected the oocyte quality and reduced the positive IVF-ET pregnancy results of women with PCOS. NADPH oxidase pathway may be involved in the mechanism of ROS production in GCs of women with PCOS.
Similar content being viewed by others
References
Lainas TG, Sfontouris IA, Zorzovilis IZ, Petsas GK, Lainas GT, Alexopoulou E, Kolibianakis EM. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: a prospective randomised controlled trial (RCT). Hum Reprod 2010; 25(3): 683–689
Orvieto R, Meltcer S, Homburg R, Nahum R, Rabinson J, Ashkenazi J. What is the preferred GnRH analogue for polycystic ovary syndrome patients undergoing controlled ovarian hyperstimulation for in vitro fertilization? Fertil Steril 2009; 91(4 Suppl): 1466–1468
Homburg R. Polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2008; 22(2): 261–274
Pal L, Zhang H, Williams J, Santoro NF, Diamond MP, Schlaff WD, Coutifaris C, Carson SA, Steinkampf MP, Carr BR, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Myers E, Legro RS; Reproductive Medicine Network. Vitamin D status relates to reproductive outcome in women with polycystic ovary syndrome: secondary analysis of a multicenter randomized controlled trial. J Clin Endocrinol Metab 2016; 101(8): 3027–3035
Klevedal C, Turkmen S. Fetal-maternal outcomes and complications in pregnant women with polycystic ovary syndrome. Minerva Ginecol 2017; 69(2): 141–149
Sabuncu T, Vural H, Harma M, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem 2001; 34(5): 407–413
Uyar A, Torrealday S, Seli E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril 2013; 99(4): 979–997
Huang B, Qian K, Li Z, Yue J, Yang W, Zhu G, Zhang H. Neonatal outcomes after early rescue intracytoplasmic sperm injection: an analysis of a 5-year period. Fertil Steril 2015; 103(6): 1432–7.e1
Adashi EY. Endocrinology of the ovary. Hum Reprod 1994; 9(5): 815–827
Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab 2001; 86(3): 1318–1323
Sharma RK, Duda T. Ca(2 +)-sensors and ROS-GC: interlocked sensory transduction elements: a review. Front Mol Neurosci 2012; 5: 42
Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H. Eight-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilizationembryo transfer program. Fertil Steril 2002; 77(6): 1184–1190
Jancar N, Kopitar AN, Ihan A, Virant Klun I, Bokal EV. Effect of apoptosis and reactive oxygen species production in human granulosa cells on oocyte fertilization and blastocyst development. J Assist Reprod Genet 2007; 24(2-3): 91–97
Rajani S, Chattopadhyay R, Goswami SK, Ghosh S, Sharma S, Chakravarty B. Assessment of oocyte quality in polycystic ovarian syndrome and endometriosis by spindle imaging and reactive oxygen species levels in follicular fluid and its relationship with IVF-ET outcome. J Hum Reprod Sci 2012; 5(2): 187–193
Huang B, Yang F, Dong X, Zheng Y, Tan H, Ai J, Jin L. Lower limit of antioxidant activity in follicular fluid: relationship to embryo quality in IVF cycle. Int J Clin Exp Med 2016; 9(8): 16346–16352
Yang CM, Lee IT, Hsu RC, Chi PL, Hsiao LD. NADPH oxidase/ ROS-dependent PYK2 activation is involved in TNF-α-induced matrix metalloproteinase-9 expression in rat heart-derived H9c2 cells. Toxicol Appl Pharmacol 2013; 272(2): 431–442
Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004; 4(3): 181–189
Huang B, Ren X, Wu L, Zhu L, Xu B, Li Y, Ai J, Jin L. Elevated progesterone levels on the day of oocyte maturation may affect top quality embryo IVF cycles. PLoS One 2016; 11(1): e0145895
Liu HC, He ZY, Mele CA, Veeck LL, Davis O, Rosenwaks Z. Human endometrial stromal cells improve embryo quality by enhancing the expression of insulin-like growth factors and their receptors in cocultured human preimplantation embryos. Fertil Steril 1999; 71(2): 361–367
Opøien HK, Fedorcsak P, Omland AK, Abyholm T, Bjercke S, Ertzeid G, Oldereid N, Mellembakken JR, Tanbo T. In vitro fertilization is a successful treatment in endometriosis-associated infertility. Fertil Steril 2012; 97(4): 912–918
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004; 89(6): 2745–2749
Balen AH. Hypersecretion of luteinizing hormone and the polycystic ovary syndrome. Hum Reprod 1993; 8(Suppl 2): 123–128
Balen AH, Tan SL, MacDougall J, Jacobs HS. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Hum Reprod 1993; 8(6): 959–964
Morais RD, Thomé RG, Lemos FS, Bazzoli N, Rizzo E. Autophagy and apoptosis interplay during follicular atresia in fish ovary: a morphological and immunocytochemical study. Cell Tissue Res 2012; 347(2): 467–478
Nakahara K, Saito H, Saito T, Ito M, Ohta N, Takahashi T, Hiroi M. The incidence of apoptotic bodies in membrana granulosa can predict prognosis of ova from patients participating in in vitro fertilization programs. Fertil Steril 1997; 68(2): 312–317
Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab 2008; 93(3): 881–887
Brown ZA, Louwers YV, Fong SL, Valkenburg O, Birnie E, de Jong FH, Fauser BC, Laven JS. The phenotype of polycystic ovary syndrome ameliorates with aging. Fertil Steril 2011; 96(5): 1259–1265
Sagle M, Bishop K, Ridley N, Alexander FM, Michel M, Bonney RC, Beard RW, Franks S. Recurrent early miscarriage and polycystic ovaries. BMJ 1988; 297(6655): 1027–1028
Saller S, Merz-Lange J, Raffael S, Hecht S, Pavlik R, Thaler C, Berg D, Berg U, Kunz L, Mayerhofer A. Norepinephrine, active norepinephrine transporter, and norepinephrine-metabolism are involved in the generation of reactive oxygen species in human ovarian granulosa cells. Endocrinology 2012; 153(3): 1472–1483
Saller S, Kunz L, Berg D, Berg U, Lara H, Urra J, Hecht S, Pavlik R, Thaler CJ, Mayerhofer A. Dopamine in human follicular fluid is associated with cellular uptake and metabolism-dependent generation of reactive oxygen species in granulosa cells: implications for physiology and pathology. Hum Reprod 2014; 29(3): 555–567
Víctor VM, Espulgues JV, Hernández-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infect Disord Drug Targets 2009; 9(4): 376–389
Karuputhula NB, Chattopadhyay R, Chakravarty B, Chaudhury K. Oxidative status in granulosa cells of infertile women undergoing IVF. Syst Biol Reprod Med 2013; 59(2): 91–98
Kim YM, Cho M. Activation of NADPH oxidase subunit NCF4 induces ROS-mediated EMT signaling in HeLa cells. Cell Signal 2014; 26(4): 784–796
Murillo I, Henderson LM. Expression of gp91phox/Nox2 in COS-7 cells: cellular localization of the protein and the detection of outward proton currents. Biochem J 2005; 385(3): 649–657
Casbon AJ, Allen LA, Dunn KW, Dinauer MC. Macrophage NADPH oxidase flavocytochrome B localizes to the plasma membrane and Rab11-positive recycling endosomes. J Immunol 2009; 182(4): 2325–2339
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81401268).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lai, Q., Xiang, W., Li, Q. et al. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front. Med. 12, 518–524 (2018). https://doi.org/10.1007/s11684-017-0575-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-017-0575-y