Abstract
To support the development of eco-friendly hybrid perovskite solar cells, structural, thermal, and physical properties of the lead-free hybrid perovskite [NH3(CH2)3NH3]CuBr4 were investigated using X-ray diffraction (XRD), differential scanning calorimetry, thermogravimetric analysis, and nuclear magnetic resonance spectroscopy. The crystal structure confirmed by XRD was monoclinic, and thermodynamic stability was observed at approximately 500 K without any phase transition. The large changes in the 1H chemical shifts of NH3 and those in C2 close to N are affected by N–H∙∙∙Br hydrogen bonds because the structural geometry of CuBr4 changed significantly. The 1H and 13C spin–lattice relaxation times (T1ρ) showed very similar molecular motions according to the Bloembergen–Purcell–Pound theory at low temperatures; however, the 1H T1ρ values representing energy transfer were about 10 times lesser than those of 13C T1ρ. Finally, the 1H and 13C T1ρ values of [NH3(CH2)3NH3]MeBr4 (Me = Cu, Zn, and Cd) were compared with those reported previously. 1H T1ρ was affected by the paramagnetic ion of the anion, while 13C T1ρ was affected by the MeBr4 structure of the anion; 13C T1ρ values in Me = Cu and Cd with the octahedral MeBr6 structure had longer values than those in Me = Zn with the tetrahedral MeBr4 structure. We believe that these detailed insights on the physical properties will play a crucial role in the development of eco-friendly hybrid perovskite solar cells.
Similar content being viewed by others

Introduction
The development of solar cells based on CH3NH3PbX3 (X = Cl, Br, and I) type organic–inorganic hybrid materials have advanced recently. However, due to the presence of Pb, these perovskites decompose in humid air and are toxic. Therefore, the development of alternative eco-friendly hybrid perovskite solar cells is crucial1,2,3,4,5. The synthesis of novel groups of organic–inorganic materials, as well as improved functional materials, has attracted significant attention. The fabrication of hybrid perovskites has recently been reported as a major challenge in the context of developing ferroelastic semiconductors6. On the other hand, the success of single-crystal-level ferroelectric performance makes hybrid perovskites suitable candidates for flexible and wearable devices7,8. As an eco-friendly alternative to sunlight, the need for detailed characterization of perovskite structures and dynamics of the new organic–inorganic hybrid compounds [NH3(CH2)nNH3]MeX4 (Me = Mn, Fe, Co, Cu, and Cd, n = 2, 3 …) with various configurations is increasing in relation to their potential applications in photovoltaic performance9,10,11,12,13,14,15,16,17,18. Perovskites comprising [NH3(CH2)nNH3] and MeX4-layered metal-halogen anionic sublattices are an interesting group of hybrid materials16,19,20,21,22,23,24,25. Their physicochemical properties are related to their structure and the interaction between the organic and inorganic components. The organic cations are related to the structural flexibility and optical properties, and the inorganic anions are related to the thermal and mechanical properties26. For Me = Mn, Cu, or Cd, the structure is two-dimensional and comprises a corner-shared octahedral (MeX6)2− with alternating organic layers. When Me = Co or Zn, the structures are tetrahedral (MeX4)2− groups sandwiched between layers of organic cations and are zero-dimensional9,16,20,27. In [NH3(CH2)nNH3]MeX4, the N–H···X hydrogen bonding occurs between the NH3 groups at both ends of the organic chains and X group of the perovskite-type layer. Among these materials, [NH3(CH2)3NH3]CuBr4 [bis (propylene-1, 3-diammonium) tetrabromocuprate] with n = 3, Me = Cu, and X = Br has a monoclinic structure with the P21/n space group at room temperature. The unit cell dimensions are a = 8.086 Å, b = 7.566 Å, c = 17.622 Å, β = 96.75°, and Z = 428. The structural geometry of [NH3(CH2)3NH3]CuBr4 is shown in Fig. 1 (CCDC: 1278590)28. The structure is composed of puckered layers of CuBr6 octahedra separated by layers of [NH3(CH2)3NH3]2+ chains that are nearly perpendicular to the layers. At both ends of the cation, ammonium groups were located between the layers. Extensive N–H···Br type hydrogen bonding was found between the Cu and Br layers and the alkylammonium chain. The organic chains extended along the crystallographic c-direction.
Several reports have been published on related materials. For example, Snively et al.29 reported the two-halide linear super-exchange bridge of the [NH3(CH2)3NH3]CuBr4 crystal, and Straatman et al.30 discussed the theoretical analysis of double-halide super-exchange. Kite and Drumheller31 conducted an electron paramagnetic resonance study of this crystal in a temperature range below the room temperature. Kallel et al.32 determined structure of [NH3(CH2)3NH3]ZnBr4 for Me=Zn by X-ray diffraction (XRD) analysis. The [NH3(CH2)3NH3]CdBr4 crystal structure for Me=Cd has been reported by XRD analysis at room temperature, and the temperature dependence of the 79,81Br nuclear quadrupole resonance spectrum near the phase transition temperatures was studied by Ishihara et al.33,34 Recently, our group reported the results for [NH3(CH2)3NH3]MeBr4 (Me=Zn and Cd) single crystal studies27,35; physicochemical properties and structural dynamics were mainly studied from the chemical shifts and spin-lattice relaxation times using nuclear magnetic resonance (NMR).
The structure and lattice constant of the [NH3(CH2)3NH3]CuBr4 single crystal grown in this study was confirmed by XRD. To understand the role of inorganic anions on the thermodynamic properties, differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) experiments were performed. In addition, the role of the organic cation in the structural properties was considered in detail using the magic angle spinning (MAS) NMR method. The chemical shifts and spin-lattice relaxation times T1ρ are discussed for 1H and 13C. In particular, the N–H···Br hydrogen bond between the Cu and Br layer and the cation within [NH3(CH2)3NH3]CuBr4 is expected to provide important characteristics for the development of perovskite material based solar cells. Finally, the physical properties of [NH3(CH2)3NH3]MeBr4 (Me=Cu, Zn, and Cd) crystals were compared with the previous reports and explained based on the paramagnetic ion and the structure of the MeX4 anion.
Methods
Materials
[NH3(CH2)3NH3]CuBr4 crystals were prepared with molecular weights of NH2(CH2)3NH2·2HBr and CuBr2 with 1:1 ratio in an aqueous solution. The mixture was stirred and heated to obtain a homogeneous solution. The resulting solution was filtered and brown colored single crystals were obtained by slow evaporation over few weeks. The crystals grew into rectangular shapes with dimensions of 5 × 5 × 1 mm3.
Characterization
The structure of the [NH3(CH2)3NH3]CuBr4 crystal at 298 K was determined by single-crystal XRD system (Bruker D8 Venture, Germany) with Mo-Kα radiation at the Western Seoul Center of the Korea Basic Science Institute (KBSI). DSC (TA Instruments, DSC 25, USA) was performed at a heating rate of 10 °C/min in the 190-600 K temperature range in a N2 gas atmosphere. Thermogravimetric analysis (TGA) was conducted using a thermogravimetric analyzer (TA Instruments, USA) in the 300–680 K temperature range at the same heating rate. Optical observations were performed using a Carl Zeiss microscope equipped with a Linkam THM-600 heating stage.
NMR spectra of the [NH3(CH2)3NH3]CuBr4 crystals were collected on a Bruker Avance II+ NMR spectrometer at the same KBSI center. The Larmor frequencies for the 1H NMR and 13C NMR experiments were 400.13 and 100.61 MHz, respectively. NMR chemical shifts were referenced to tetramethylsilane (TMS) as the standard. The powdered sample in the cylindrical zirconia rotor was spun at a rate of 10 kHz in the MAS NMR experiment. The spin-locking field during the 1H and 13C NMR acquisition was 71.42 kHz. The 1H T1ρ and 13C T1ρ values were measured by changing the spin-locking pulse duration. 1H and 13C NMR data were measured using a single pulse sequence with a pulse width of 3.25–3.59 μs. NMR data could not be measured at temperatures above 430 K because NMR equipment that could measure at such high temperatures are unavailable.
Experimental results
Crystal structure
Single-crystal XRD analysis of [NH3(CH2)3NH3]CuBr4 was performed at 298 K. The structure, lattice constants, and space group of this crystal were monoclinic, with a = 8.052 ± 0.009 Å, b = 7.560 ± 0.009 Å, c = 17.611 ± 0.190 Å, β = 96.920 ± 0.05°, Z = 4, and P21/n, and this result was in good agreement with a previous report by Halvorson and Willett28.
Thermodynamic property
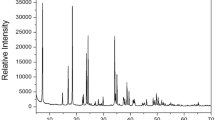
In the DSC experiment, there was no phase transition temperature in the 200–500 K range; however, large exothermic peaks were observed at 546 and 577 K (Supplementary Information 1). To confirm that the DSC peaks at 546 and 577 K are related to the phase transition, TGA and differential thermal analysis (DTA) experiments were performed; the results are shown in Fig. 2. The TGA results revealed that this crystal is thermally stable up to 504 K. The initial weight loss of [NH3(CH2)3NH3]CuBr4 began at 504 K, and there was no weight loss before the decomposition temperature. In the TGA curve, [NH3(CH2)3NH3]CuBr4 exhibited a two-stage decomposition at high temperatures. The initial weight loss (17%) occurred in the 500–550 K range, which may be due to the decomposition of HBr in [NH3(CH2)3NH3]CuBr4. The second-stage decomposition (63%) occurred because of the presence of an inorganic moiety in the 550–650 K range. The amount that remained as a solid was calculated from the TGA data and chemical reactions. The weight losses of 17 and 35% at approximately 546 and 607 K are likely due to the decomposition of the HBr and 2HBr moieties, respectively, which is consistent with the exothermic peak in the DSC curve. The molecular weight decreased abruptly between 550 and 650 K, with a corresponding weight loss of 63% at approximately 650 K.
Further, optical polarizing microscopy experiments were conducted to understand the thermal decomposition and melting phenomena of the crystals. The crystal was brown at 300 K, as shown in the inset of Fig. 2. While no changes were observed from 300 to 500 K, the crystal began to melt slightly and changed from brown to dark brown at approximately 543 K. The color change was likely due to decomposition from the loss of HBr, and also due to the geometrical changes in CuBr4. Above 600 K, the surface and edges melted significantly. The thermogram clearly indicated that 543 K was the melting point of the crystal. Hence, the [NH3(CH2)3NH3]CuBr4 crystal is suitable for applications up to 504 K.
1H NMR chemical shifts and spin–lattice relaxation times
The 1H NMR chemical shifts of the [NH3(CH2)3NH3]CuBr4 crystals were recorded while increasing the temperature, as shown in Fig. 3. Below 270 K, only one 1H resonance signal was observed, and the intensity and line-width of the 1H signal were very small and wide, respectively, making the detection challenging. The resonance signal exhibited asymmetric shapes owing to the overlapping of the two types of NH3 and CH2 signals. The 1H NMR chemical shift for CH2 was recorded at δ = 5.51 ppm at 300 K, whereas that for NH3 was recorded at δ = 10.94 ppm, which subsequently split into two resonance lines. The spinning sidebands for CH2 are represented by open circles and those for NH3 are represented by crosses. The 1H chemical shifts for CH2, shown by the dotted line in Fig. 3, did not significantly change with increasing temperature, whereas the change in the 1H chemical shifts for NH3 toward the lower chemical shift as the temperature increased. The greater shift in the 1H NMR chemical shift of the NH3 in the cation with changes in the temperature, than that of the CH2 is reason for the large change in the N−H∙∙∙Br hydrogen bonding between the Br around Cu and the H of NH3.
The relationship of the decay rate of proton magnetization is defined by T1ρ and the Eq. (1)27,36,37,38,39
where P(τ) and P(0) are the signal intensities at times τ and τ = 0, respectively. The intensity changes observed in the 1H NMR spectra were recorded with changing delay times at a given temperature, and at 300 K, the 1H NMR spectrum was plotted with a delay time ranging from 0.01 to 30 ms as shown in Fig. 4. From the slope of the intensities of the 1H signal indicated by the arrow vs. delay times, the 1H T1ρ could be calculated using Eq. (1). As a result, the 1H T1ρ values for CH2 and NH3 are shown in Fig. 5 as a function of the inverse temperature. The 1H T1ρ values were in the order of a few milliseconds for CH2 and NH3, and their values were temperature-dependent. As shown in the cation structure in Fig. 5, the 1H of CH2 is expressed in red, and the 1H of NH3 is expressed in black, which is the same as the T1ρ values. The T1ρ values decreased as the temperature increased and then increased sharply again at 210 K. Below 300 K, only the 1H T1ρ value for NH3 is shown, and the 1H T1ρ values of CH2 above 300 K have longer T1ρ values than 1H of NH3. The T1ρ vs. inverse temperature curve showed a minima of 5.80 ms at 210 K, which indicates the existence of distinct molecular motions. The T1ρ values can be explained by the correlation time τC for the molecular motion. The T1ρ value for the molecular motion based on the Bloembergen–Purcell–Pound (BPP) theory is given by35,36,37,38,39:
where Fa = τC/[1 + ω12τC2], Fb = τC/[1 + (ωC − ωH)2τC2], Fc = τC/[1 + ωC2τC2], Fd = τC/[1 + (ωC + ωH)2τC2], and Fe = τC/[1 + ωH2τC2]. Here, G is a coefficient, γH and γC are the gyromagnetic ratios of the proton and carbon, respectively, ħ is the reduced Planck constant, ωH and ωC are the Larmor frequencies of 1H and 13C, respectively, r is the distance between the proton and carbon, and ω1 is the spin-locking pulse sequence with a locking pulse of 71.42 kHz. In the rotating frame, τC can be obtained when ω1τC = 1, and the coefficient G in Eq. (2) can be obtained from T1ρ, ωH, ωC, and ω1. Using this G value, τC was obtained as a function of inverse temperature. The local field fluctuation is governed by the thermal motion, which is activated by thermal energy. Therefore, τC is represented by the Arrhenius behavior: τC = τoexp(− Ea/kBT), where Ea and kB are the activation energy and Boltzmann constant, respectively36. The τC vs. 1000/T was plotted on a logarithmic scale (inset of Fig. 5), and the Ea of 1H, depending on the molecular dynamics, was obtained using 4.25 ± 0.25 kJ/mol by dot line.
13C NMR chemical shifts and spin–lattice relaxation times
The 13C NMR chemical shifts of [NH3(CH2)3NH3]CuBr4 were measured as a function of temperature, as shown in Fig. 6. The 13C MAS NMR spectra exhibited two resonance signals at all temperatures. The 13C NMR spectrum for TMS was recorded at 38.3 ppm at 300 K and was used to determine the exact chemical shift of 13C27. Here, the CH2 between the two CH2 groups is labeled C1, and the CH2 close to NH3 is labeled C2, as shown in the inset of Fig. 6. The two resonance signals at 300 K were recorded at chemical shifts of δ = 33.54 and δ = 177.07 ppm for C1 and C2, respectively. The 13C chemical shifts for CH2 were different for C1 far from those of NH3 and C2 close to that of NH3. The 13C chemical shift for C1 changed slowly and did not vary significantly with increasing temperature, whereas that for C2 moved abruptly to the lower chemical-shift side with increasing temperature compared to that for C1.
The changes in the intensities of the 13C NMR spectral peaks with increasing delay times were measured at a given temperature in the same manner as the 1H T1ρ measurement method. The 13C T1ρ values from the slope of the recovery traces are described by a single exponential function in Eq. (1). The T1ρ values for C1 and C2 as a function of 1000/temperature are shown in Fig. 7. In the cation structure, C1 is shown in green, C2 is shown in red, and T1ρ is shown in the same way. 13C T1ρ values for C2 showed no changes in the temperature range measured in this study, and the T1ρ for C1 according to the temperature change showed a similar trend as that for 1H T1ρ. The 13C T1ρ values were approximately 10 times longer than the 1H T1ρ values. The 13C T1ρ values were unaffected by the spin diffusion owing to the small dipolar coupling, which results from the low natural abundance. On the other hand, the T1ρ values decreased as the temperature increased, and then increased again at 200 K. Below 300 K, only the 13C T1ρ value for C1 vs. the inverse temperature showed a minimum of 28.58 ms at 200 K, which implies the existence of active molecular motions at low temperatures. τC values on a logarithmic scale, as obtained by Eq. (2) vs. 1000/T, were plotted (inset of Fig. 7). The Ea, depending on the molecular dynamics of 13C, was measured to be 8.59 ± 0.47 kJ/mol. The 13C Ea value was approximately twice that of 1H Ea.
Discussion
1H and 13C NMR T1ρ values in [NH3(CH2)3NH3]MeBr4 (Me=Cu, Zn, and Cd) crystals were compared and discussed with previously reported results27,35. In single crystals of [NH3(CH2)3NH3]MeBr4 (Me=Cu, Zn, and Cd), the crystal structure, phase transition temperature, and T1ρ for 1H and 13C when the cation lengths are the same and the Me is different, are shown in Table 1. The changes in the chemical shifts of both 1H and 13C for three crystals with temperature are shown in Supplementary Information 2 and 3. When Zn and Cd were included, the 1H and 13C chemical shifts were similar, whereas when Cu was included, the chemical shifts were different. The differences in the chemical shifts are due to the differences in the electron structures of the metal ions, Me. Cu2+ has one electron, outside the closed d-shell. Zn2+ and Cd2+ have two electrons outside its closed shell37.
1H T1ρ values with a paramagnetic Cu2+ ion have a very different trend from those of Zn2+ and Cd2+ without the paramagnetic ion; Zn and Cd show a very similar trend and indicate temperature dependence alone (see Fig. 8). T1ρ has a very less value because of the Cu2+-containing paramagnetic ions. Because T1ρ is inversely proportional to the square of the magnetic moment of the paramagnetic Cu2+ ions, the value is in the order of a few milliseconds. Unlike the 1H T1ρ values, the 13C T1ρ values when Zn is included are in the order of 10 ms, and the 13C T1ρ values when Cu and Cd are included have a very long order of 100 ms, and are sensitive to temperature, as shown in Fig. 9. From these results, the effect of paramagnetic ions was directly affected by 1H, which is close to that of Cu, but 13C T1ρ was not significantly affected by the paramagnetic ions. In addition, the structures of Cu and Cd are related to the octahedral MeBr6, and the structure of Zn is related to tetrahedral MeBr4. The 13C T1ρ values in [NH3(CH2)3NH3]CuBr4 and [NH3(CH2)3NH3]CdBr4 with the two-dimensional octahedral MeBr6 structure have longer values than those in [NH3(CH2)3NH3]ZnBr4 with the zero-dimensional structure of the tetrahedral MeBr4. The 1H T1ρ is affected by the paramagnetic ion of the cation, and the 13C T1ρ is thought to be affected by the MeX4 or MeX6 structure of the anion.
Conclusion
To investigate the structural, thermal, and physical properties of the lead-free hybrid perovskite [NH3(CH2)3NH3]CuBr4 crystal, we performed XRD, DSC, TGA, optical polarizing microscopy, and NMR spectroscopy. First, the monoclinic structure of this crystal was confirmed by XRD, and its thermodynamic stability was observed at approximately 500 K without phase transition. Second, the NMR chemical shifts were due to the local field around the resonating nucleus in the crystals, and the chemical shifts in the 1H and 13C NMR spectra indicated changes in the crystallographic geometry. The 1H chemical shifts of NH3 changed significantly with temperature compared to those of the CH2 because the structural environment around the 1H of NH3 changed to a greater degree than the structural environment around the 1H of CH2. In addition, the 13C chemical shifts for C1 increased slowly with increasing temperature, whereas the chemical shifts for C2 shifted significantly compared to C1. Consequently, the large changes in the 1H chemical shifts of NH3 and the chemical shift of C2 close to N were affected by the N−H∙∙∙Br hydrogen bonds owing to the extensive changes in the structural geometry of CuBr4. Finally, 1H and 13C T1ρ showed very similar trends for temperature changes; however, the 1H T1ρ values were approximately 10 times shorter than the 13C T1ρ values. T1ρ values show that the energy transfer, with a large thermal displacement around the 1H atoms of the cation, is very short. Ea values for 1H and 13C at low temperatures were 4.25 and 8.59 kJ/mol, respectively, indicating that the value for 1H was smaller.
The detailed elucidation of these physical properties is expected to greatly facilitate the development of potential eco-friendly material applications. In the future, we plan to study [NH3(CH2)3NH3]MeI4, which may be more suitable candidate for solar cells. Furthermore, as the most popular approaches to materials design, we plan to expand and study a high-quality film and relevant characterizations.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Hermes, I. M. et al. Ferroelastic fingerprints in methylammonium lead iodide perovskite. J. Phys. Chem. C 120, 5724 (2016).
Abdel-Aal, S. K., Abdel-Rahman, A. S., Kocher-Oberlehner, G. G., Ionov, A. & Mozhchil, R. Structure, optical studies of two-dimensional hybrid perovskite for photovoltaic applications. Acta Cryst. A 70, C1116 (2017).
Strelcov, E. et al. CH3NH3PbI3 perovskites: ferroelasticity revealed. Sci. Adv. 3, e1602165 (2017).
Liu, Y. et al. Chemical nature of ferroelastic twin domains in CH3NH3PbI3 perovskite. Nat. Mater. 17, 1013 (2018).
Lee, J., Lee, W., Kang, K., Lee, T. & Lee, S. K. Layer-by layer structural identification of 2D ruddlesden-popper hybrid lead iodide perovskites by solid-state NMR spectroscopy. Chem. Mater. 33, 370 (2021).
Su, C. et al. Hybrid optical-electrical perovskite can be a ferroelastic semiconductor. CCS Chem. 3, 2021 (2021).
Xie, Y. et al. The soft molecular polycrystalline ferroelectric realized by the fluorination effect. J. Am. Chem. Soc. 142, 12486 (2020).
Fu, D.-W. et al. High-TC enantiometric ferroelectric based on homochiral dabco-derivatives (Dabco=1,4-Diazabicyclo[2.2.2.]octane. Angew. Chem. Int. Ed. 59, 17477 (2020).
Gonzalez-Carrero, S., Galian, R. E. & Perez-Prieto, J. Organometal halide perovskites: bulk low-dimension materials and nanoparticles. Part. Syst. Charact. 32, 709 (2015).
Abdel-Adal, S. K., Kocher-Oberlehner, G., Ionovand, A. & Mozhchil, R. N. Effect of organic chain length on structure, electronic composition, lattice potential energy, and optical properties of 2D hybrid perovskites [(NH3)(CH2) n (NH3)]CuCl4, n = 2–9. Appl. Phys. A 123, 531 (2017).
Liu, W. et al. Giant two-photon absorption and its saturation in 2D organic-inorganic perovskite. Adv. Opt. Mater. 5, 1601045 (2017).
Staskiewicz, B. & Staskiewicz, A. The influence of the relative thermal expansion and electric permittivity on phase transitions in the perovskite-type bidimensional layered NH3(CH2)3NH3CdBr4 compound. J. Phys. Chem. Solids 106, 65 (2017).
Czupinski, O., Ingram, A., Kostrzewa, M., Przeslawski, J. & Czapla, Z. On the structural phase transition in a perovskite-type diaminopropanetetrachlorocuprate(II) NH3(CH2)3NH3CuCl4 crystal. Acta Phys. Pol., A 131, 304 (2017).
Elseman, M. et al. Copper-substituted lead perovskite materials constructed with different halides for working (CH3NH3)2CuX4-based perovskite solar cells from experimental and theoretical view. ACS Appl. Mater. Interfaces 10, 11699 (2018).
Aramburu, J. A., Garcia-Fernandez, P., Mathiesen, N. R., Garcia-Lastra, J. M. & Moreno, M. Changing the usual interpretation of the structure and ground state of Cu2+ layered perovskites. J. Phys. Chem. C 122, 5071 (2018).
Przeslawski, J., Czapla, Z., Crofton, M. & Dacko, S. On the “inverted” phase transitions in ferroic crystals containing propylenediammonium cations. Ferroelectrics 534, 220 (2018).
Wang, Y. et al. (1,4-Butyldiammonium)CdBr4: a layered organic–inorganic hybrid perovskite with a visible-blind ultraviolet photoelectric response. Inorg Chem Front 5, 2450 (2018).
Liang, D., Lian, X., Li, X. & Luo, B. Pb alloying enables efficient broadband emission of two dimensional [NH3(CH2)4NH3]CdBr4. J. Solid State Chem. 293, 121772 (2021).
Mitzi, D. B. Templating and structural engineering in organic-inorganic perovskites. J. Chem. Soc. Dalton Trans 1, 1 (2001).
Saikumar, S., Ahmad, J. J., Baumberg, G. & Vijaya Prakash, G. Fabrication of excitonic luminescent inorganic-organic hybrid nano- and microcrystals. Scr. Mater. 67, 834 (2012).
Ahmad, S., Hanmandlu, C., Kanaujia, P. K. & Vijaya Prakash, G. Direct deposition strategy for highly ordered inorganic organic perovskite thin films and their optoelectronic applications. Opt. Mater. Express 4, 1313 (2014).
Wei, Y., Audebert, P., Galmiche, L., Lauret, J. S. & Deleporte, E. Photostability of 2D organic–inorganic hybrid perovskites. Materials 7, 4789 (2014).
Chen, Q. et al. Under the spotlight: the organic–inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 10, 355 (2015).
Svane, K. L. et al. How strong is the hydrogen bond in hybrid perovskites?. J. Phys. Chem. Lett. 8, 6154 (2017).
Lim, A. R. & Kim, S. H. Physicochemical property investigations of perovskite-type layer crystals [NH3(CH2)nNH3]CdCl4 (n = 2, 3, and 4) as a function of length n of CH2. ACS Omega 6, 27568 (2021).
Zang, W. & Xiong, R.-G. Ferroelectric metal–organic frameworks. Chem. Rev. 112, 1163 (2012).
Lim, A. R., Kim, S. H. & Joo, Y. L. Physicochemical properties and structural dynamics of organic–inorganic hybrid [NH3(CH2)3NH3]ZnX4 (X = Cl and Br) crystals. Sci. Rep. 11, 8408 (2021).
Halvorson, K. & Willett, R. D. Structures of ethylenediammonium tetrabromocuprate (II) and propylenediammonium tetrabromocuprate (II). Acta Cryst. C 44, 2071 (1988).
Snively, L. O., Haines, D. N., Emerson, K. & Drumheller, J. E. Two-halide superexchange in [NH3(CH2)nNH3]CuBr4 for n=3 and 4. Phys. Rev. B 26, 5245 (1982).
Straatman, P., Block, R. & Jansen, L. Theoretical analysis of double-halide superexchange in layered solids of the compounds [NH3(CH2)nNH3]CuBr4 with n=3 and 4. Phys. Rev. B 29, 1415 (1984).
Kite, T. & Drumheller, J. E. Electron paramagnetic resonance of the eclipsed layered compounds NH3(CH2)nNH3CuBr4 with n = 2, 3, 4. J. Mag. Reson. 54, 253 (1983).
Kallel, A., Fail, J., Fuess, H. & Daoud, A. 1,3-propanediammonium tetrachlorozincate (II). Acta Cryst. B 36, 2788 (1980).
Ishihara, H. et al. Isolated versus condensed anion structure II; the influence of the cations (1,3-propanediammonium, 1,4-phenylendiammonium, and n-propylammonium) on structures and phase transitions of CdBr 2–4 salts A 79,81Br NQR and X-ray structure analysis. Z. Naturforsch. A: Phys. Sci. 51, 1216 (1996).
Ishihara, H. et al. NQR, NMR, and DSC study of [NH3(CH2)3NH3]CdBr 4. Chem. Lett. 25, 371–372 (1996).
Lim, A. R. Thermal, ferroelastic, and structural properties near phase transitions of organic–inorganic perovskite type [NH3(CH2)3NH3]CdBr4 crystals. RSC Adv. 11, 17622 (2021).
Abragam, A. The Principles of Nuclear Magnetism (Oxford University Press, 1961).
Koenig, J. L. Spectroscopy of Polymers (Elsevier, 1999).
Harris, R. K. Nuclear Magnetic Resonance Spectroscopy (Pitman Pub., London, 1983).
Bloembergen, N., Purcell, E. M. & Pound, R. V. Relaxation effects in nuclear magnetic resonance absorption. Phys. Rev. 73, 679 (1948).
Acknowledgements
This research was supported by the Basic Science Research program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2018R1D1A1B07041593 and 2016R1A6A1A03012069).
Author information
Authors and Affiliations
Contributions
A.R.L. designed the project and performed. N.M.R. experiments. L.K.K. performed. D.S.C. and T.G.A. experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lim, A.R., Kwac, L.K. Advances in physicochemical characterization of lead-free hybrid perovskite [NH3(CH2)3NH3]CuBr4 crystals. Sci Rep 12, 8769 (2022). https://doi.org/10.1038/s41598-022-12832-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12832-y
- Springer Nature Limited