Abstract
We analyzed the implantation effects on cruciate ligament force in unicompartmental knee arthroplasty (UKA) and determined whether kinematics is associated with the cruciate ligament force. We examined 16 patients (17 knees) undergoing medial UKA. Under fluoroscopy, each participant performed a deep knee bend before and after UKA. A two-dimensional/three-dimensional registration technique was employed to measure tibiofemoral kinematics. Forces in the anteromedial and posterolateral bundles of both the anterior cruciate ligament (aACL and pACL) and the anterolateral and posteromedial bundles of the posterior cruciate ligament (aPCL and pPCL) during knee flexion were analyzed pre- and post-UKA. Correlations between changes in kinematics and ligament forces post-UKA were also analyzed. Preoperatively, the aACL forces were highly correlated with anteroposterior (AP) translation of the lateral condyles (Correlation coefficient [r] = 0.59). The pPCL forces were highly correlated with the varus–valgus angulation (r = − 0.57). However, postoperatively, the PCL forces in both bundles were highly correlated with the AP translation of the medial femoral condyle (aPCL: r = 0.62, pPCL: r = 0.60). The ACL and PCL forces of the knees post-UKA were larger than those of the knees pre-UKA. Kinematic changes were significantly correlated with the cruciate ligament force changes.
Similar content being viewed by others
Introduction
Unicompartmental knee arthroplasty (UKA) is a procedure that is associated with a high level of patient satisfaction among patients with osteoarthritis (OA). Several studies have reported that more patients are satisfied with UKA than with total knee arthroplasty (TKA)1,2. In addition, some studies have demonstrated a greater rate of return to sports activities after UKA than after TKA3,4. The knee kinematics observed following UKA have been shown to be closer to normal than those following bicruciate-retaining TKA (BCR-TKA)5. Furthermore, the kinematics following UKA generally remain similar to preoperative knee kinematics6,7, except for the varus–valgus angulation6. A previous study has reported that preoperative and postoperative femurs displayed external rotation with flexion, and that the anteroposterior (AP) translation of the medial and lateral sides indicated posterior movement with flexion6. The difference between the medial and lateral sides of both preoperative and postoperative knees following UKA represented a medial pivot pattern from 0° to 50° of flexion and from 50° to 130° of flexion, with observed posterior rollback6. There were no significant differences in the femoral rotation angles, AP translations, and kinematic pathways in the mid-flexion range of motion before and after UKA6. In contrast, preoperative UKA knees showed a significant varus alignment from 10° to 60° of flexion, compared with postoperative knees6.
One of the reasons near-normal kinematics are observed in UKA knees may be due to the preservation of the anterior cruciate ligament (ACL) and posterior cruciate ligament (PCL) during this procedure. In normal knees, the ACL is shortened with flexion, while the PCL extends during flexion8,9. In contrast, several studies have demonstrated that the cruciate ligament force following BCR-TKA was higher than that of normal knees10,11. Moreover, the postoperative ACL force was higher than that observed preoperatively. However, the cruciate ligament force of UKA knees remains unknown.
Several studies have reported that the knee kinematics among patients with OA are different from normal knee kinematics12,13. Therefore, a patient’s preoperative osteoarthritic condition might affect postoperative knee kinematics. However, the association between kinematics and cruciate ligament force before and after UKA is largely unknown. Moreover, the independent relation between kinematics and the cruciate ligament force remains unknown as well.
Numerous people perform high knee flexion activities, such as gardening or exercising. Several studies have reported that participating in high knee flexion activities is related to better clinical outcome, patient satisfaction, and patient expectations after knee joint replacement14,15. Therefore, we aimed to evaluate high knee flexion activities within the scope of this study.
This study was designed to analyze the effects of implantation on the cruciate ligament force in UKA and to determine whether kinematics is associated with the cruciate ligament force. We hypothesized that implantation would affect and increase the cruciate ligament force. This is because there is a significant difference in the varus–valgus angulation between before and after UKA, and a previous study has demonstrated cruciate ligament elongation after coronal realignment in TKA16.
Results
Kinematic changes
The preoperative and postoperative knees were flexed from 4.1 ± 7.4° to 134.9 ± 14.3°, and from 3.8 ± 6.0° to 129.1 ± 15.2°, respectively, on average. There were no significant differences in the minimum and maximum flexion angle between the preoperative and postoperative values (p = 0.11 and 0.89 in the minimum and maximum flexion, respectively).
Preoperatively, the femurs displayed steep external rotation (8.0 ± 5.1°) relative to the tibia from 10° to 50° of flexion, reaching 19.5 ± 4.2° on average. Postoperatively, the femurs displayed steep external rotation (4.9 ± 4.2°) relative to the tibia from 10° to 50° of flexion, reaching 17.3 ± 6.9° on average. There were no significant differences between the preoperative and postoperative knees (p = 0.48) (Fig. 1).
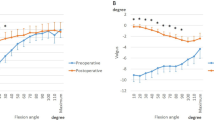
The preoperative knees showed 1.4 ± 4.1° varus movement up to 50° of flexion, while the postoperative knees showed no significant movement. From 10° to 60° of flexion, the preoperative knees indicated a significant varus position compared to the postoperative knees (Fig. 2).
The preoperative and postoperative AP translation of the medial side indicated 10.8 ± 16.0% and 18.1 ± 12.1% posterior movement with flexion, respectively. There was no significant difference between the preoperative and postoperative values (p = 0.44) (Fig. 3).
The preoperative and postoperative AP translation of the lateral side indicated 46.7 ± 20.2% and 41.7 ± 19.3% posterior movement with flexion, respectively. There was no significant difference between the preoperative and postoperative values (p = 0.28) (Fig. 3).
ACL forces
The femoral and tibial attachment areas of the anteromedial and posterolateral bundles of the ACL (aACL and pACL) decreased with flexion both preoperatively and postoperatively (Figs. 4, 5). At 40° to 60° of flexion, the postoperative aACL force was larger than the preoperative one.
PCL forces
The anterolateral and posteromedial bundles of the PCL (aPCL and pPCL) increased with flexion both preoperatively and postoperatively (Figs. 6, 7). At 50° to 110° of flexion, the postoperative aPCL force was larger than the preoperative one. In addition, at 10° to 110° of flexion, the postoperative pPCL force was larger than the preoperative one.
Correlation between kinematics and the cruciate ligament force
Linear regression was used to determine whether knee kinematic patterns were associated with ligament tensile forces (Table 1). Preoperatively, the aACL forces were highly correlated with the AP translation of the lateral condyles. In addition, the pPCL forces were highly correlated with the varus–valgus angulation. However, postoperatively, the PCL forces in both bundles were highly correlated with the AP translation of the medial femoral condyle.
Discussion
The most important findings of this study were that the knees of the patients with OA showed a larger cruciate ligament force following UKA than that before UKA, and the cruciate ligament force was correlated with the varus–valgus angulation and AP translation.
Mochizuki et al. have reported that UKA does not completely recreate normal knee kinematics7. However, a previous study has demonstrated that the kinematics following UKA are more similar to the normal knee kinematics than those following BCR-TKA5. Furthermore, the kinematics before UKA resembled those following UKA in terms of axial rotation and AP translation; although the preoperative knees indicated a significant varus alignment compared with the postoperative knees6. In this study, the postoperative ACL and PCL forces were larger than the preoperative values. These facts suggest that coronal realignment drives the re-tensioning of cruciate ligaments.
Valgus angulation was positively correlated with the aACL force during postoperative squatting; however, it was negatively correlated with the pPCL force during preoperative squatting. This suggests that valgus angulation with flexion postoperatively increases the aACL force, and severe varus angulation preoperatively increases the pPCL. Specifically, the excessive postoperative valgus angulation might result in a non-physiological ligament force. Hopgood et al. have reported that a 2-mm increase in insert thickness achieved a correction of 1.5°–2.9°17. Further, Walker et al. have reported that updated instrumentation appears to be an effective tool in determining an adequate level of tibial resection, which, in turn, prevents unnecessary bone loss and reduces the need for thicker bearings18. The bearing thickness in this study was 4.0 ± 0.9 mm. Therefore, the bearing thickness might affect the coronal alignment and cruciate ligament force. This is because such a large bearing thickness might drive the valgus angulation and subsequently increase the aACL force.
The lateral AP translation was positively correlated with the aACL force during preoperative squatting. The medial and lateral AP translations were positively correlated with both the aPCL and pPCL forces during postoperative squatting. This suggests that reduced posterior translation on the lateral femoral condyle induces ACL tightness preoperatively, and reduced femoral rollback from mid-flexion to high-flexion induces PCL tightness postoperatively.
According to a study, the ACL force in the postoperative knees was greater than the force in the preoperative knees following BCR-TKA; this is similar to our present findings19. Okada et al. demonstrated that the in situ ACL force against 100 N of anterior force in BCR-TKA knees was statistically comparable with that of intact knees at all flexion angles10. Furthermore, Sabouret et al. have reported that TKA with retention of the ACL remained functional and provided adequate stability at 22 years of follow-up20. In TKA, sacrificing the ACL reportedly achieved inferior clinical outcomes21. Moreover, compared to posterior cruciate-retaining (PCR)-TKA, BCR-TKA achieved more normal-like kinematics22. Finally, it has been reported that normal-like kinematics promote favorable clinical outcomes23. These facts suggest that ACL retention in UKA drives a strongly favorable clinical outcome.
Although there was no significant difference in the PCL force in BCR-TKA19, the PCL force in the postoperative UKA knees was larger than that in the preoperative knees. Tsai et al. have reported that the PCL in BCR-TKA was significantly overstretched in deep flexion positions, resonating with a previous posterior cruciate-retaining TKA study16, which reported overstretching of the PCL in PCR-TKA during deep flexion and attributed this to reduced femoral rollback24. The PCL in UKA might be overstretched. In addition, overstretching of the PCL might increase the ligament force. However, the PCL tension in UKA may be larger than that in TKA, even though the PCL in both UKA and TKA is elongated.
Malalignment of the lower limb following UKA is associated with a poorer clinical outcome25. Since no ligament release is performed in UKA, a precise osteotomy could result in preservation of the appropriate ligament balance. Several previous studies have reported that navigation- and robotic-assisted UKAs improve the accuracy and survival following surgery26,27. To improve osteotomy accuracy and for achieving appropriate ligament balance, a widespread use of navigation- and robotic-assisted UKAs may be important.
This study has some limitations. First, we did not evaluate normal knees since there were no patients with non-arthritic contralateral knees included in the study. Therefore, the difference between UKA knees and normal knees remains unclear. Second, this study cohort had a relatively short mean follow-up duration of 9.5 months. The kinematics and ligament force at long-term follow-up may differ from those reported in this study. Third, the relationship between soft tissue balance and cruciate ligament force remains unclear since the gap was evaluated based on a feel-gauge rather than quantitatively. Fourth, in this study, only mobile-bearing UKA was analyzed. Several studies have reported that the kinematics in mobile-bearing UKA is different from that of fixed-bearing UKA6,28. Therefore, our findings may not be representative of fixed-bearing UKA. Fifth, only patients with OA were included in the study. Therefore, the findings may not be representative of patients with osteonecrosis. Sixth, only patients who could perform squatting activities were included in the current study; therefore, our findings may not be generalizable to those who cannot perform such activities.
In conclusion, the ACL and PCL forces of the knees following UKA were larger than those of the knees before UKA. Moreover, the kinematic changes were significantly correlated with changes in the cruciate ligament forces.
Methods
We investigated a total of 16 patients (17 knees) who underwent medial UKA (Oxford partial knee, Zimmer Biomet G.K., Warsaw, USA). Patients who suffered from medial OA but could perform squatting activities were included. The patients provided informed consent to participate in this investigation, which was approved by the institutional review board (provided by The University of Tokyo Institutional Ethics Review Board).
The following methods were carried out in accordance with the relevant guidelines and regulations. All surgeries were performed using a minimally invasive approach to comply with the Oxford Group recommendations29,30. Using a sagittal saw blade aimed towards the hip center, a vertical tibial incision was made at the medial edge of the anterior cruciate ligament insertion on the tibia. Subsequently, a horizontal incision was made using the tibial saw guide, which had a 7° built-in posterior slope set parallel to the long axis of the tibia in the coronal and sagittal planes. Femoral drilling was performed using an Oxford Microplasty device (MP: Biomet Ltd., Swindon, UK) to facilitate reproducible implantation18. Following these procedures, we performed a gap balancing procedure between knee flexion and extension and a modified keel cutting method as previously reported31. With the trial component and bearing in place, the knee was manipulated through a full range-of-motion (ROM) to demonstrate joint stability, security of the mobile-bearing joint, and absence of impingement.
The surgeries were performed by five knee surgeons, and a highly experienced surgeon (TT or HI) oversaw all procedures as either the chief surgeon or first assistant.
Each patient was asked to perform a squatting activity. Fluoroscopic surveillance was performed in the sagittal plane while each patient performed a squatting motion at a natural pace. The patients practiced the motion several times before the recording. Knee motions were recorded before (within 1 month) and after (at least 6 months) the UKA. At the postoperative fluoroscopic analysis, the patients’ mean age was 73.2 ± 6.5 years. The patients’ mean body height was 154.6 ± 9.7 cm and their mean body weight was 57.9 ± 7.7 kg. The mean duration of postoperative follow-up was 9.5 ± 2.3 months. Among the 16 patients (17 knees) included in the analysis, 6 were men and 11 were women. All patients underwent UKA to treat medial knee joint OA (Kellgren and Lawrence grade III). The mean hip–knee–ankle angles at the time of analysis were 172.6° ± 3.1° preoperatively and 174.8° ± 3.1° postoperatively6.
The sequential motion was recorded as digital X-ray images (1024 × 1024 × 12 bits/pixel, 7.5-Hz serial spot images as a DICOM file) using a 17-inch flat panel detector system (C-vision Safire L, Shimadzu, Kyoto, Japan and ZEXIRA DREX-ZX80, Toshiba, Tokyo, Japan). All images were processed using dynamic range compression to enable edge-enhanced images. To estimate the spatial position and orientation of the knee, a two-dimensional (2D)/three-dimensional (3D) registration technique was used32,33. This technique is based on a contour-based registration algorithm that uses single-view fluoroscopic images and 3D computer-aided design (CAD) models32,33. We created 3D bone models using computed tomography (CT) before surgery and used them for CAD models. The estimation accuracy for relative motion between 3D bone models was ≤ 1° in rotation and ≤ 1 mm in translation33. The local coordinate system in the bone model was produced according to a previous study33. Knee rotations were described using the joint rotational convention of Grood and Suntay34. The femoral rotation and varus–valgus angle relative to the tibia, and the AP translation of the medial sulcus (medial side) and lateral epicondyle (lateral side) of the femur on the plane perpendicular to the tibial mechanical axis during each flexion angle were evaluated6,33. AP translation was calculated as a percentage relative to the proximal AP dimension of the tibia6,33. External rotation was denoted as positive, and internal rotation as negative. Valgus was defined as positive, and varus as negative. Positive or negative values of AP translation were defined as anterior or posterior to the axis of the tibia, respectively.
Both bundles of the ACL and PCL were identified using the osseous landmarks on the preoperative CT and magnetic resonance imaging (MRI)8,35,36,37. The accuracy of the attachment area was within 0.7 ± 0.1 mm37. Each cruciate ligament force was calculated using commercially available software (VivoSim, Advanced Mechanical Technology Inc., Watertown, MA, USA). The path of each ligament was assumed to be a straight line, and the effects of the ligament-bone contact were neglected. Each ligament was assumed to be elastic, and its properties were described using a nonlinear force–strain curve38,39,40. The stiffness values and reference lengths of the model ligaments were based on the data reported by Shelburne et al.40,41; the properties of the model ligaments were adjusted to match the measurements of the intact knee-joint laxity and ACL-deficient knees obtained from previous cadaver studies39,42. The ligament stiffnesses assumed in the model are shown in Table 2. The cruciate ligament forces at each flexion were evaluated (Video 1). To investigate whether ligament forces were related to the knee kinematics, we computed the changes in femoral rotation, varus–valgus angulation, and AP position of the femoral condyles, as well as changes in ligament forces for each ligament bundle from 10° to 120° of flexion6. We then evaluated the correlation between the change in each kinematic parameter and the change in force for each cruciate ligament bundle. AP translation was calculated as a percentage of the proximal AP dimension of the tibia6. External rotation was denoted as positive, and internal rotation was denoted as negative. Valgus angulation was defined as positive, whereas varus was defined as negative. Positive or negative values of AP translation were defined as anterior or posterior to the axis of the tibia, respectively. All values were expressed as means ± standard deviations.
Statistical analyses
The results were analyzed using IBM Corp. (Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). Repeated-measures analysis of variance (ANOVA) and post-hoc pairwise comparison (Bonferroni test) were used to analyze all evaluation items and compare for multiple comparisons. Pearson’s correlation coefficient was used to analyze the correlation between differences in knee kinematics and the corresponding differences in cruciate ligament force. Statistical significance was set at p ≤ 0.05. Moreover, a power analysis using Easy R (EZR)43 indicated that a dataset with data from nine knees would be required for an alpha set at 0.05, and statistical power set at 0.8.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Liddle, A. D., Pandit, H., Judge, A. & Murray, D. W. Patient-reported outcomes after total and unicompartmental knee arthroplasty: A study of 14,076 matched patients from the National Joint Registry for England and Wales. Bone Jt. J. 97, 793–801. https://doi.org/10.1302/0301-620x.97b6.35155 (2015).
Von Keudell, A. et al. Patient satisfaction after primary total and unicompartmental knee arthroplasty: An age-dependent analysis. Knee 21, 180–184. https://doi.org/10.1016/j.knee.2013.08.004 (2014).
Hopper, G. P. & Leach, W. J. Participation in sporting activities following knee replacement: Total versus unicompartmental. Knee Surg. Sports Traumatol. Arthrosc. 16, 973–979. https://doi.org/10.1007/s00167-008-0596-9 (2008).
Witjes, S. et al. Return to sports and physical activity after total and unicondylar knee arthroplasty: A systematic review and meta-analysis. Sports Med. (Auckland, N. Z.) 46, 269–292. https://doi.org/10.1007/s40279-015-0421-9 (2016).
Kono, K. et al. Bicruciate-retaining total knee arthroplasty reproduces in vivo kinematics of normal knees to a lower extent than unicompartmental knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 28, 3007. https://doi.org/10.1007/s00167-019-05754-2 (2019).
Kono, K. et al. In vivo kinematic comparison before and after mobile-bearing unicompartmental knee arthroplasty during high-flexion activities. Knee 27, 878–883. https://doi.org/10.1016/j.knee.2020.03.002 (2020).
Mochizuki, T. et al. Kinematics of the knee after unicompartmental arthroplasty is not the same as normal and is similar to the kinematics of the knee with osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 22, 1911–1917. https://doi.org/10.1007/s00167-013-2767-6 (2014).
Kono, K. et al. In vivo length change of ligaments of normal knees during dynamic high flexion. BMC Musculoskelet. Disord. 21, 552. https://doi.org/10.1186/s12891-020-03560-3 (2020).
Rao, Z. et al. In vivo kinematics and ligamentous function of the knee during weight-bearing flexion: An investigation on mid-range flexion of the knee. Knee Surg. Sports Traumatol. Arthrosc. 28, 797. https://doi.org/10.1007/s00167-019-05499-y (2019).
Okada, Y. et al. ACL function in bicruciate-retaining total knee arthroplasty. J. Bone Joint Surg. Am. 100, e114. https://doi.org/10.2106/jbjs.18.00099 (2018).
Tsai, T. Y. et al. Bi-Cruciate retaining total knee arthroplasty does not restore native tibiofemoral articular contact kinematics during gait. J. Orthop. Res. 37, 1929. https://doi.org/10.1002/jor.24333 (2019).
Hamai, S. et al. Knee kinematics in medial osteoarthritis during in vivo weight-bearing activities. J. Orthop. Res. 27, 1555–1561. https://doi.org/10.1002/jor.20928 (2009).
Kawashima, K. et al. In vivo three-dimensional motion analysis of osteoarthritic knees. Mod. Rheumatol. 23, 646–652. https://doi.org/10.1007/s10165-012-0703-0 (2013).
Noble, P. C., Conditt, M. A., Cook, K. F. & Mathis, K. B. The John Insall Award: Patient expectations affect satisfaction with total knee arthroplasty. Clin. Orthop. Relat. Res. 452, 35–43. https://doi.org/10.1097/01.blo.0000238825.63648.1e (2006).
Nakahara, H. et al. Correlations between patient satisfaction and ability to perform daily activities after total knee arthroplasty: Why aren’t patients satisfied? J. Orthop. Sci. 20, 87–92. https://doi.org/10.1007/s00776-014-0671-7 (2015).
Yue, B., Varadarajan, K. M., Rubash, H. E. & Li, G. In vivo function of posterior cruciate ligament before and after posterior cruciate ligament-retaining total knee arthroplasty. Int. Orthop. 36, 1387–1392. https://doi.org/10.1007/s00264-011-1481-6 (2012).
Hopgood, P., Martin, C. P. & Rae, P. J. The effect of tibial implant size on post-operative alignment following medial unicompartmental knee replacement. Knee 11, 385–388. https://doi.org/10.1016/j.knee.2003.12.008 (2004).
Walker, T. et al. The influence of different sets of surgical instrumentation in Oxford UKA on bearing size and component position. Arch. Orthop. Trauma Surg. 137, 895–902. https://doi.org/10.1007/s00402-017-2702-2 (2017).
Kono, K. et al. In vivo kinematics and cruciate ligament forces in bicruciate-retaining total knee arthroplasty. Sci. Rep. 11, 5645. https://doi.org/10.1038/s41598-021-84942-y (2021).
Sabouret, P., Lavoie, F. & Cloutier, J. M. Total knee replacement with retention of both cruciate ligaments: A 22-year follow-up study. Bone Jt. J. 95, 917–922. https://doi.org/10.1302/0301-620x.95b7.30904 (2013).
Jacobs, C. A., Christensen, C. P. & Karthikeyan, T. An intact anterior cruciate ligament at the time of posterior cruciate ligament-retaining total knee arthroplasty was associated with reduced patient satisfaction and inferior pain and stair function. J. Arthroplasty 31, 1732–1735. https://doi.org/10.1016/j.arth.2016.01.026 (2016).
Smith, L. A. et al. In vivo knee kinematics: How important are the roles of femoral geometry and the cruciate ligaments? J. Arthroplasty 36, 1445–1454. https://doi.org/10.1016/j.arth.2020.10.020 (2021).
Van Onsem, S., Verstraete, M., Van Eenoo, W., Van Der Straeten, C. & Victor, J. Are TKA kinematics during closed kinetic chain exercises associated with patient-reported outcomes? A preliminary analysis. Clin. Orthop. Relat. Res. 478, 255–263. https://doi.org/10.1097/corr.0000000000000991 (2020).
Tsai, T. Y. et al. In-vivo elongation of anterior and posterior cruciate ligament in bi-cruciate retaining total knee arthroplasty. J. Orthop. Res. 36, 3239. https://doi.org/10.1002/jor.24132 (2018).
Inui, H. et al. Prosthetic alignment and clinical outcomes of navigation-assisted unicompartmental knee arthroplasty by an experienced surgeon compared with inexperienced surgeons. J. Arthroplasty 36, 2435–2439. https://doi.org/10.1016/j.arth.2021.02.053 (2021).
Weber, P. et al. Improved accuracy in computer-assisted unicondylar knee arthroplasty: A meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 21, 2453–2461. https://doi.org/10.1007/s00167-013-2370-x (2013).
Chowdhry, M., Khakha, R. S., Norris, M., Kheiran, A. & Chauhan, S. K. Improved survival of computer-assisted unicompartmental knee arthroplasty: 252 cases with a minimum follow-up of 5 years. J. Arthroplasty 32, 1132–1136. https://doi.org/10.1016/j.arth.2016.11.027 (2017).
Kono, K. et al. Weight-bearing status affects in vivo kinematics following mobile-bearing unicompartmental knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 29, 718–724. https://doi.org/10.1007/s00167-020-05893-x (2021).
Goodfellow, J. W., Kershaw, C. J., Benson, M. K. & O’Connor, J. J. The Oxford Knee for unicompartmental osteoarthritis. The first 103 cases. J. Bone Jt. Surg. Br. 70, 692–701. https://doi.org/10.1302/0301-620x.70b5.3192563 (1988).
Kawaguchi, K. et al. Intraoperative mobile-bearing movement in Oxford unicompartmental knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 27, 2211–2217. https://doi.org/10.1007/s00167-018-5064-6 (2018).
Inui, H. et al. A modified technique to reduce tibial keel cutting errors during an Oxford unicompartmental knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 25, 710–716. https://doi.org/10.1007/s00167-016-4151-9 (2017).
Yamazaki, T. et al. Improvement of depth position in 2-D/3-D registration of knee implants using single-plane fluoroscopy. IEEE Trans. Med. Imaging 23, 602–612 (2004).
Kono, K. et al. In vivo three-dimensional kinematics of normal knees during different high-flexion activities. Bone Jt. J. 100, 50–55. https://doi.org/10.1302/0301-620x.100b1.bjj-2017-0553.r2 (2018).
Grood, E. S. & Suntay, W. J. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. J. Biomech. Eng. 105, 136–144 (1983).
Inou, Y., Tomita, T., Kiyotomo, D., Yoshikawa, H. & Sugamoto, K. What kinds of posterior cruciate ligament bundles are preserved in cruciate-retaining total knee arthroplasty? A three-dimensional morphology study. J. Knee Surg. 32, 989–994. https://doi.org/10.1055/s-0038-1675184 (2018).
Shino, K., Mae, T. & Tachibana, Y. Anatomic ACL reconstruction: Rectangular tunnel/bone-patellar tendon-bone or triple-bundle/semitendinosus tendon grafting. J. Orthop. Sci. 20, 457–468. https://doi.org/10.1007/s00776-015-0705-9 (2015).
Lee, Y. S., Seon, J. K., Shin, V. I., Kim, G. H. & Jeon, M. Anatomical evaluation of CT-MRI combined femoral model. Biomed. Eng. Online 7, 6. https://doi.org/10.1186/1475-925x-7-6 (2008).
Blankevoort, L. & Huiskes, R. Ligament-bone interaction in a three-dimensional model of the knee. J. Biomech. Eng. 113, 263–269 (1991).
Pandy, M. G., Sasaki, K. & Kim, S. A three-dimensional musculoskeletal model of the human knee joint. Part 1: Theoretical construct. Comput. Method. Biomech. 1, 87–108. https://doi.org/10.1080/01495739708936697 (1998).
Shelburne, K. B., Kim, H. J., Sterett, W. I. & Pandy, M. G. Effect of posterior tibial slope on knee biomechanics during functional activity. J. Orthop. Res. 29, 223–231. https://doi.org/10.1002/jor.21242 (2011).
Shelburne, K. B. & Pandy, M. G. A musculoskeletal model of the knee for evaluating ligament forces during isometric contractions. J. Biomech. 30, 163–176. https://doi.org/10.1016/s0021-9290(96)00119-4 (1997).
Shelburne, K. B., Pandy, M. G., Anderson, F. C. & Torry, M. R. Pattern of anterior cruciate ligament force in normal walking. J. Biomech. 37, 797–805. https://doi.org/10.1016/j.jbiomech.2003.10.010 (2004).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48, 452–458. https://doi.org/10.1038/bmt.2012.244 (2013).
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
K.K. contributed to formal analysis, investigation, and writing of the manuscript. H.I., S.K. and T.K. carried out data curation and conceived the study. T.Y. provided technical assistance. R.Y., K.K., S.S., T.T., S.T., D.D. and S.T. provided general support. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kono, K., Inui, H., Tomita, T. et al. Cruciate ligament force of knees following mobile-bearing unicompartmental knee arthroplasty is larger than the preoperative value. Sci Rep 11, 18233 (2021). https://doi.org/10.1038/s41598-021-97655-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97655-z
- Springer Nature Limited