Abstract
In the present study, 199 pre-selected Carbohydrate-Active enZymes (CAZymes) and sulfatases were assessed, either alone or in combination, to evaluate their capacity to disrupt Laminaria digitata cell wall, with the consequent release of interesting nutritional compounds. A previously characterized individual alginate lyase, belonging to the family 7 of polysaccharide lyases (PL7) and produced by Saccharophagus degradans, was shown to be the most efficient in the in vitro degradation of L. digitata cell wall. The alginate lyase treatment, compared to the control, released up to 7.11 g/L of reducing sugars (p < 0.001) and 8.59 mmol/100 g dried alga of monosaccharides (p < 0.001), and reduced cell wall fluorescence intensity by 39.1% after staining with Calcofluor White (p = 0.001). The hydrolysis of gel-forming polymer alginate by the alginate lyase treatment could prevent the trapping of fatty acids and release beneficial monounsaturated fatty acids, particularly 18:1c9 (p < 0.001), to the extracellular medium. However, no liberation of proteins (p > 0.170) or pigments (p > 0.070) was observed. Overall, these results show the ability of an individual alginate lyase, from PL7 family, to partially degrade L. digitata cell wall under physiological conditions. Therefore, this CAZyme can potentially improve the bioavailability of L. digitata bioactive compounds for monogastric diets, with further application in feed industry.
Similar content being viewed by others
Introduction
Due to an increasing interest in the use of macroalgae for food and feedstuffs1, as well as for pharmaceutical industries, organic fertilizers, eutrophication inhibition, bioremediation and biogas generation1, their cultivation has been steadily growing over the last decade. The nutritional profile of macroalgae, although variable among species and depending on growth location and harvesting season1, consists of numerous vitamins, minerals, pigments, phenolic compounds, carbohydrates and high quality proteins1. Carbohydrates comprise a high proportion of macroalgae biomass (from 4 to 76% dry matter, DM)2, whereas lipids are usually found in small amounts (< 5% DM) with values up to 1.13% DM in brown algae3. However, their lipid profile can be rich in monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids4,5,6, which might have beneficial effects on human health7.
Laminaria sp. are seawater multicellular eukaryotic and autotrophic brown macroalgae, which are amongst the most cultivated seaweeds worldwide, representing the largest biomass in coastal regions8. Brown macroalgae have a distinct carbohydrate-rich cell-wall9,10, which comprises up to 45% DM of alginate and fucose-containing sulphated polysaccharides (FCSPs) (fucans or homofucans and fucoidans or heterofucans), as well as small amounts of cellulose (1 to 8% DM)11, β-1,3 glucans, unbranched mixed-linkage β-D-glucans (1,3-and-1,4-β-D-glucose residues) masked by alginate12 and arabinogalactans linked to proteins13. The proportion of alginates, fucoidans and cellulose was found to be 3:1:110. Alginate is a linear polysaccharide composed of only two epimers, β-1,4-D-mannuronic (M) and α-1,4-L-guluronic (G) acids, arranged in heteropolymeric (MG) or homopolymeric (MM or GG) blocks along the polymer chain11. FCSPs are composed of two different backbones with α-1,3- or alternating α-1,3-/α-1,4-linked L-fucose residues. These motifs form acetylated or branched structures presenting one to three sulphate esters on positions O-2, O-3 or O-411. Laminarin is a polysaccharide located in intracellular vacuoles that constitutes the carbon storage of macroalgae and is composed of (1,3)-β-D-glucopyranose residues with some 6-O-branching in the main chain and β-1,6-intrachain links14,15. These polysaccharides were found to have bioactive properties with relevance for potential applications in functional foods and feeds, cosmetics and pharmaceutical products13,14,15. For instance, alginate was shown to reduce blood pressure and cholesterol and to have antimicrobial and anticancer activities15; FCSPs were described as antioxidant, anti-inflammatory, immune-stimulant, antimicrobial and anticancer compounds13,15; and laminarin was reported as an anticancer, anti-inflammatory, immunostimulatory, anticoagulant and antioxidant14,15 agent.
However, cell wall carbohydrates are organized in a complex cross-linked matrix, resulting in a highly recalcitrant cell wall that resists breakage and serves as a natural defence mechanism for algae9. In fact, alginate cross-links with phenolic compounds and constitutes gel-forming and hydroscopic polymers that control cell wall rigidity11. These polymers form a network that embed fucose-containing sulphated polysaccharides. The latter are tightly assembled to cellulose microfibrils by cross-linkage11. The intricate macroalga cell walls have been described to exert anti-nutritional effects for monogastric animals, by trapping valuable nutrients, with a concomitant decrease in the efficiency of feed digestion and absorption16. The presence of complex polysaccharides in seaweed cell walls can also decrease the rate of algae biomass hydrolysis during the production of renewable energies, thus reducing bioethanol and biogas yields17.
Mechanical processes, such as hammer mill, are usually applied for incorporation of seaweed in diets for monogastric animals1. However, mechanical methods are less specific for macroalgae cell disruption than enzymatic procedures. Therefore, the latter can be more advantageous for the release of algae valuable nutritional and bioactive compounds and increase of their bioavailability18. The effectiveness of exogenous enzymes (i.e. cellulases, xylanases19,20,21,22 and a mixture of carbohydrases18) on hydrolysing algae biomass with an increase of protein extraction or digestibility was previously demonstrated for green (i.e. Ulva rigida)18 and red (e.g. Palmaria palmata, Gracilaria sp. and Chondrus sp.)19,20,21,22 seaweeds. Other studies reported the use of cellulases, alginate lyases23,24,25 and a carbohydrase mixture26 for the degradation of brown macroalgae biomass (Laminaria digitata23,25, Saccharina latissima24,25 and a mixture of different species including Sargassum sp.26) envisaging biotechnological applications. These applications consisted in the production of bioethanol and biogas23,25, algae saccharification24 and extraction of bioactive compounds26.
Therefore, exogenous Carbohydrate-Active enzymes (CAZymes) could be a suitable option to deconstruct macroalgae cell wall, similarly to what was recently described by our research team for microalgae27,28. Moreover, these exogenous enzymes were shown to improve the nutritional value of cereal-based diets29,30, with the consequent industrial application as feed additives for poultry and pigs31. Additionally, the use of sulfatases could be of major importance for the degradation of the recalcitrant structure of branched and sulphated FCSPs, as reported in a recent study32. Thus, we hypothesised that CAZymes and sulfatases could, individually or in combination, degrade the recalcitrant L. digitata cell wall, with the consequent improvement of nutrients bioavailability. Cell wall disruption was assessed by fluorescence microscopy, reducing sugars and oligosaccharides profile after incubation of macroalga with the enzymes. The release of nutritive and bioactive compounds from macroalga, following the enzyme treatment, was assessed by quantifying proteins, pigments and fatty acids.
Results
CAZymes and sulfatases selection and evaluation of expression and purity of recombinant enzymes
All of the CAZYmes and sulfatases selected for the initial screen were chosen for their activity, either determined or predicted, towards specific compounds of brown macroalgae cell walls, like alginate, FCSFs and cellulose11. Furthermore, most are produced by marine halophilic bacteria, which are organisms likely adapted to feed on algae biomass. The catalytic activity and the biochemical properties of the majority of the candidates were previously described in the literature and their amino acid sequences can be accessed in Genbank (see Supplementary Table S1). Seventeen of the selected CAZYmes (ID 166 to 174, 184, 189 to 195) did not have their activity and substrate specificity previously characterized, but were selected due to sharing high homology (see Supplementary Table S1 for Genbank accession numbers) to some of the other well-characterized candidates.
In order to evaluate the soluble protein yield for each enzyme, a qualitative scale was used based on the protein concentration (g/L): -, 0.0; + , 0.1 > 2.1; + + , 2.1 > 4.1; + + + , 4.1 > 6.1; + + + + , > 6.1 (see Supplementary Table e S1). From the 199 recombinant enzymes, 23 did not express (-), 81 had low expression levels or were mostly insoluble ( +) and 95 had good expression and solubility levels (+ + , + + + , + + + +). Among low expressing enzymes, 4 were slightly degraded (ID 5, 69, 73 and 129). Among high expressing enzymes, 1 had low solubility, 1 was degraded and 3 (ID 147, 151 and 184) had a different SDS-PAGE migration pattern than what was expected from the calculated molecular weight (data not shown). All soluble protein fractions were enriched by a high throughput IMAC protocol before the activity screens (see Supplementary Fig. S1).
Screening of individual enzymes for Laminaria digitata cell wall disruption
Each one of the 176 CAZymes and sulfatases with low to high expression levels (see Supplementary Table S1) was individually incubated with a macroalga suspension in phosphate buffered saline (PBS) solution for an evaluation of their ability to degrade L. digitata cell wall. The majority of enzymes was unable to deconstruct alga biomass (see Supplementary Table S2), but 8 individual enzymes (ID 6, 18, 20, 21, 22, 28, 29 and 46) had a measurable capacity to degrade the cell wall of L. digitata, as shown in Table 1. This ability was assessed by both the release of reducing sugars, as evaluated through the 3,5-dinitrosalicylic acid (DNSA) method, and the decrease of fluorescence intensity from Calcofluor white stained cell walls. The fact that brown macroalgae cell walls form an intricate carbohydrate structure11 will allow the evaluation of cell wall integrity loss by assessing the reduction of Calcofluor white staining fluorescence intensity, even though the dye only binds specifically to minor compounds of the cell wall (cellulose and, to some extent, mixed-liked 1,3–1,4-β-glucans)12,33. Therefore, the data in Table 1 is presented according to two qualitative scales: the first scale is based on the amount of released reducing sugars (g/L): -, 0.00 < 2.77; + , 2.77 < 3.99; + + , 3.99 < 5.20; + + + , 5.20 < 6.42; and + + + + , > 6.42; whereas the second one is based on the decrease of fluorescence intensity (%): -, 0.00 < 9.92; + , 9.92 < 20.0; + + , 20.0 < 29.9; and + + + , 29.9 < 38.5; + + + + , > 38.5. For the enzymes with ID 6 and 46, the release of reducing sugars (average of 0.46 g/L) was in the lower level considered in Table 1 and they caused only a low to intermediate decrease of fluorescence intensity (up to 22.3%). However, enzyme with ID 46 was selected because its predicted substrate is α-linked L-fucopyranosyl units, which are the main residues found in one of the major components of brown seaweed cell walls, the FCSPs11. Although the substrate for the enzyme with ID 6 (mixed linked 1,3–1,4-β-glucans) is only a minor component of brown macroalgae cell walls, its insoluble nature can impact the degradation of algae cell wall, mainly through the association with a major cell wall polysaccharide, the alginate12.
Selection of the most active enzymes and assessment of their synergistic action
In order to disclose synergistic actions among individual enzymes, an eight-enzyme mixture based on the initial screening (Table 1), was compared to a three-enzyme mixture (ID 18, 22 and 46) (Table 2, Figs. 1, 2 and 3). These three enzymes were selected based on their organism of origin, thermostability and main substrate. Indeed, laminarinase (ID 18) and alginate lyase (ID 22) were isolated from marine and halophilic bacteria (Thermotoga napolitana34 and Saccharophagus degradans35, respectively), and were described as being thermoresistant with optimum catalytic activities at 85 to 95 ºC34 and 50 ºC35, respectively. Although the enzyme with ID 46 was from a non-marine and non-halophilic bacterium (Lactobacillus casei)36, it was relatively thermostable, with an optimum temperature of 42 ºC36, and acted towards a main constituent of brown algae cell wall (α-linked L-fucopyranosyl units)11.
(a) Fluorescence intensity derived from Calcofluor White staining for control assay and alginate lyase (AL; ID 22) treatment. Asterisk denotes statistical difference at p = 0.001. (b) and (c): fluorescence images (× 400) of Laminaria digitata suspension stained with Calcofluor White for control assay and alginate lyase treatment, respectively.
(a) Fluorescence intensity derived from Calcofluor White staining for control assay and laminarinase (LA; ID 18) treatment. No statistical differences were observed (p = 0.070). (b) and (c) fluorescence images (× 400) of Laminaria digitata suspension stained with Calcofluor White for control assay and laminarinase treatment, respectively.
(a) Fluorescence intensity derived from Calcofluor White staining for control assay and fucosidase (FU; ID 46) treatment. No statistical differences were observed (p = 0.195). (b) and (c) fluorescence images (× 400) of Laminaria digitata suspension stained with Calcofluor White for control assay and fucosidase treatment, respectively.
The eight-enzyme mixture led to a release of reducing sugars of 6.74 g/L, which corresponded to an increase of only 0.57 g/L compared to the three-enzyme mixture. Then, the latter mixture was compared to the activities of each enzyme composing it. It was observed that, when enzyme ID 22 enzyme was individually incubated with L. digitata suspension, the value of released reducing sugars was identical (p = 0.443) to the three-enzyme mixture (6.17 g/L). In contrast, the released reducing sugars by the other individual enzymes (4.57 g/L for enzyme ID 18, and 0.50 g/L for enzyme ID 46) were significantly lower than that of mixture (p < 0.001). Altogether, these results indicate the absence of significant (p > 0.050) additive or synergistic effects among enzymes.
The ratios of released reducing sugars were found to be: alginate lyase versus three-enzyme mixture = 101.3%; alginate lyase versus laminarinase = 136.6%, and alginate lyase versus fucosidase = 1242%. Regarding the above values, the alginate lyase ID 22 was selected as the most active enzyme for the degradation of L. digitata cell wall.
Effect of alginate lyase on Laminaria digitata cell wall integrity
The extension of released reducing sugars and decreased fluorescence pixels of stained cell walls promoted by the selected alginate lyase (ID 22; Provisional Patent number, INPI, Portugal) are presented in Table 1. The latter is also illustrated in Figs. 1a, b and c. The amount of reducing sugars (7.11 g/L) was significantly increased (p < 0.001), whereas the number of pixels (179 to 109; 39.1% decrease of fluorescence intensity) was significantly reduced (p = 0.001) with the enzyme ID 22, when compared to the control assay.
Activity, thermostability and proteolysis assays of alginate lyase
Catalytic activity of alginate lyase (ID 22) was evaluated by both UV spectroscopy, using alginate as substrate at pH 7.5 and 37 °C, and DNSA method. The enzyme showed an activity of 1.52 ± 0.026 AU/min @233 nm and 0.282 ± 0.0025 g reducing sugars/L × min.
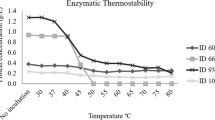
The purified (> 90% purity) alginate lyase was tested for its thermostability and proteolysis resistance. For the thermostability assay, the intact protein was subjected to a range of temperatures (30 to 80 ºC) (Fig. 4). The enzyme maintained its stability at 37 and 40 ºC. Although significant (p < 0.001), only a small variation of protein concentration was found between these two temperatures (0.81 to 0.74 g/L, respectively). However, the stability of alginate lyase declined abruptly between 40 °C and 50 ºC, with the enzyme being completely degraded at 50 ºC. The proteolytic resistance of alginate lyase is shown in Table 3 and Fig. 5. The enzyme showed partial resistance over the entire assay time.
Proteolysis assay results. Electrophoresis on SDS-PAGE in 14% (w/v) acrylamide gels displaying the fragment bands of alginate lyase (ID 22) (0.83 g/L) after incubation with pancreatin (final concentration of 2.5 g/L). The gel band of 31.8 KDa corresponds to purified protein, whereas the other bands correspond to degraded protein and pancreatin. B: blank (purified undigested ID22) P: purified protein submitted to hydrolysis by pancreatin, M: protein marker.
Effect of alginate lyase on the release of mono- and oligosaccharides from Laminaria digitata cell wall
Figure 6 shows the influence of alginate lyase treatment on the release of mono- and oligosaccharides from L. digitata cell wall. The composition of mono and oligosaccharides was a mixture of compounds, not individually identified due to its complexity and lack of some commercial standards. With the enzyme treatment, monosaccharide concentrations significantly increased (p < 0.001), from 0.02 to 8.64 mmol/100 g dried alga, in relation to the control. Although the amount of oligosaccharides did not significantly differed (p = 0.260) between assays, a numerical increase, from 1.19 to 2.57 mmol/100 g dried alga, was found for the alginate treatment. In addition, residual amounts of glucose (8.24 × 10–4 mmol/100 g dried alga) were released from L. digitata biomass with the alginate lyase treatment (data not shown).
Effect of alginate lyase on the release of proteins and pigments from Laminaria digitata biomass
The influence of alginate lyase (ID 22) treatment on pigment and protein concentrations in the supernatant and residue fractions is presented in Table 4. These results indicate if the hydrolysis of the viscous gel-like structure formed by the polymer alginate11 led to the release of trapped valuable nutrients. The incubation of alga with the enzyme did not trigger (p > 0.100) the release of protein from L. digitata cells and, thus, a similar protein content was found for the enzyme and control assays (31.1 and 39.9 mg/g alga for the supernatant, and 114 and 91.7 for the residue). Additionally, no significant differences (p > 0.071) between assays were observed for chlorophyll, carotenoid and fucoxanthin contents in both centrifugation fractions.
Effect of alginate lyase on the release of fatty acids from Laminaria digitata biomass
Fatty acid profile in residue and supernatant fractions, after incubation with the alginate lyase (ID 22), was analysed to determine if the enzyme treatment led to the release of fatty acids from L. digitata cells to the external environment (Table 4), since these nutrients could have been trapped by the gel-forming structure of alginate.
For both supernatant and residue fractions, the percentage of fatty acids were as follows: saturated fatty acids (SFA) > MUFA > PUFA > n-6 PUFA > n-3 PUFA. In the supernatant, the amount of total fatty acids was increased (p = 0.001) from 1.30 to 4.22 mg/g dried alga with the alginate lyase treatment. In fact, higher percentages of total MUFA (p < 0.001) were found in the presence of enzyme, with a major contribution of 18:1c9 (p < 0.001) and additional contributions of 16:1c9, 18:1c11 and 20:1c11. In addition, the percentage of total SFA was significantly decreased (p = 0.001) with the alginate lyase treatment, particularly of 12:0, 14:0, 16:0, 18:0, 20:0 and 22:0, as well as the amount of total n-6 PUFA (p = 0.038). The 18:2n-6 fatty acid tended to decrease (p = 0.053) with the alginate lyase treatment, although it was found in small percentages for both assays (2.08% for control and 1.35% for the enzyme).
In the residue fraction, the alginate lyase treatment caused no significant differences (p > 0.096) either in the amount of total fatty acids or in the percentage of individual fatty acids, leading only to a significant decrease of total SFA (p = 0.037) comparatively to the control.
Discussion
A large library of 176 CAZymes and 23 sulfatases was produced to test the hypothesis that some of these enzymes, with well-characterized biochemical characteristics (see Supplementary Table S1), could disrupt the recalcitrant cell wall of L. digitata with the consequent increase of nutrients availability. The production of enzymes was done in a high-throughput (HTP) platform and consisted of several steps, including gene synthesis, gene cloning, recombinant protein expression in E. coli cells and protein purification. These 199 enzymes were selected based on the polysaccharide matrix composition of macroalga cell wall, which comprises mainly alginate and fucose-containing sulphated polysaccharides, as well as minor amounts of cellulose, putative hemicellulose11 and mixed-linked β-glucans12. Laminarin13,14,15 is the main carbon storage of brown seaweeds. In addition, the enzyme origin was also attended in the selection, being 121 of them from marine and halophilic bacteria and 41 from thermophilic or hyperthermophilic bacteria.
An individual screening of the enzymes was performed in order to assess their ability to degrade L. digitata cell wall, which was evaluated by measuring the release of reducing sugars and the intensity of microscopic fluorescence. Afterwards, the 8 selected recombinant enzymes (see Table 1) were combined and tested in order to obtain a maximum disruption of L. digitata cell wall, and then reduced to a combination of 3 enzymes (laminarinase ID 18, alginate lyase ID 22 and α-L-fucosidase ID 46). However, the alginate lyase led to a degradation of macroalga cell wall similar to the simultaneous use of the 3 enzymes. The absence of synergistic and additive effects between enzymes indicates that, as previously reported for brown macroalgae11, the different polysaccharides of L. digitata cell wall, namely alginate and FCSFs, are not interlinked within the cell wall structure.
This enzyme (ID 22) belongs to the family of polysaccharide lyase 7 (PL7) and is produced by S. degradans35. It possesses poly-β-mannuronate (EC 4.2.2.3) and, to a lesser extent, poly-β-guluronate (EC4.2.2.11) lyase activities. The alginate lyase endolytically depolymerize alginate by β-elimination, having both alginate and oligo-alginates as its main substrates35. This enzyme (ID 22) showed to be resistant to proteolysis and thermostable until 40 ºC, with an abrupt decrease of thermostability between 40 and 50 ºC. These results are likely due to the tertiary structure of protein that confers both thermotolerance and an inherent proteinase resistance37. The instability of this enzyme at high temperatures (> 50 ºC) over an increasing period of time was already reported35, even though maximum catalytic activity was described as 50 ºC35. This aspect might be explained by the fact that S. degradans is a mesophilic, instead of a thermophilic, Gram-negative bacterium. However, this organism is one of the strongest marine biomass degraders, being capable of hydrolysing a great variety of polysaccharides38.
Furthermore, the efficiency of the recombinant mannuronate-specific alginate lyase from PL7 family (ID 22) on releasing reducing sugars (7.11 g/L) from brown macroalga cell wall was firstly demonstrated herein. In fact, only one previous study25 reported the recovery of reducing sugars from L. digitata and S. latissima biomass (10 and 11 g/L, respectively), through the action of a commercial mannuronate-specific alginate lyase, belonging to the PL5 family (Genbank accession numbers: SUJ15243.1 and SUJ21107.1). This enzyme was produced by Sphingobacterium multivorum during the enzymatic pre-treatment of algae biomass for biogas production. However, in the present study, a well-characterized endotype PL5 alginate lyase (ID 41) produced by Sphingomonas sp. A1 (Genbank accession number BAB03312.1), which specifically acts on poly-β-mannuronate regions (EC 4.2.2.3) and depolymerizes alginate into tri- and disaccharides39, was significantly (p < 0.050) less efficient at releasing reducing sugars (1.70 g/L) than the 3 analysed enzymes from PL7 family (average of 6.07 g/L). The release of reducing sugars with alginate lyase (ID 22) is possibly related to an increase of monosaccharides and, although not significant, of oligosaccharides released from L. digitata biomass40. Although a recombinant alginate lyase with the same catalytic site as the enzyme with ID 22 was previously shown to produce unsaturated oligoalginates (degrees of polymerization of 2 to 5) but not monosaccharides35,40, a mixture of unidentified monosaccharides, which was probably composed of uronic acid residues but needs further analysis, was released by the enzyme treatment (ID 22) in the present study. The fact that residual amounts of glucose (8.24 × 10–4 mmol/100 g dried macroalga) were obtained with the alginate lyase treatment might indicate that the breaking down of viscous-gel like matrix of alginate lyase (ID 22) released trapped laminarin from the intracellular algae compartment, which became available for a possible autohydrolysis, as previously reported23. Although, to date, a selective effect of alginate lyase towards carbohydrates from brown seaweed cell wall was never reported, two studies evaluated the release of glucose from brown macroalgae biomass during either bioethanol production23 or algae saccharification24. The first described a slight release of glucose from L. digitata biomass, by using a commercial mannuronate-specific alginate lyase, from an unknown family, produced by Sphingobacterium spiritivorum. The latter, similarly to the present study, reported no effect of 3 recombinant alginate lyases from PL7 family on the release of glucose from S. latissima.
In the present study, the decrease of cell wall fluorescence intensity (39.1%) promoted by alginate lyase (ID 22) indicates a partial alga cell wall degradation, similarly to what was observed by our research team for microalgae27,28. Considering that Calcofluor white preferentially binds to cellulose and, to some extent, mixed-liked 1,3–1,4-β-glucans in macroalgae cell walls rather than to acidic polysaccharides12,33, like alginate and FCSPs in brown algae11, the degradation of alginate seems to have compromised the whole integrity of the intricate macroalgae cell wall structure with a decreased dye binding by cellulose and mixed-liked glucans. The cell wall disruption was likely due to the ability of enzyme with ID 22 to degrade polymannuronate residues of alginate and consequently compromise cell wall rigidity that is controlled by the gel-forming structure alginate11. In fact alginate from L.digitata cell wall was found to be mainly composed of mannuronic acid (M) instead of guluronic acid (G) residues (M/G ratio between 1.99 and 3.0)41. The preferential activity of alginate lyases from PL7 family on polymannuronic acid rather than on polyguluronic acid or alginate was demonstrated in a recent study42 when the enzyme produced by a marine fungus Paradendryphiella salina was incubated with 3 different brown alga species (Ascophyllum nodosum, S. latissima and Fucus serratus). However, the activity of PL7 alginate lyase (ID 22) is not specific for polymannuronate as this enzyme can also act on polyguluronate residues of alginate (Kim et al., 2012). Conversely, the catalytic residues of enzyme with ID 41 (Genbank accession number: Q9KWU1) were shown to strictly bind to mannuronic acid residues39. This latter aspect can help to explain why PL7 alginate lyase (ID 22) was more efficient on degrading L. digitata cell wall than the PL5 alginate lyase (ID 41), in the present study.
Conversely to carbohydrates, alginate lyase (ID 22) did not release (hydro-) soluble proteins from L. digitata cells to the external environment. These results can be due to the extracellular presence of phenolic compounds (i.e. phlorotannins) previously cross-linked to alginates11, which have protein-linkage properties43, and hydrocolloidal anionic polysaccharides (e.g. oligoalginates)44. These compounds would limit the access and quantification of proteins, through the increased viscosity of extraction medium, a phenomenon that was indeed observed in the supernatants from alginate lyase treatment, and reported for other carbohydrases37. Their presence was already found to be a limiting factor of protein extraction when cellulase and xylanase acted on P. palmata19,21,45. In addition, no release of pigments from L. digitata cells to the extracellular medium was found with the alginate lyase treatment. The amounts of pigments in control assay residues were slightly different from the values (mg/g dried alga) previously reported for Laminaria sp.46 (0.124 versus 0.184 for chlorophyll a, 0.006 versus 0.014 for chlorophyll b, 0.034 versus 0.026 for total carotenoids), which was probably due to the use of different solvents for pigment extraction46, as well as variations on alga species and harvesting season47. Similarly to what was reported for microalgae27,28, the alginate lyase (ID 22) could not disrupt the long parallel lamellae of tree thylakoids in cytoplasmic plastids of brown seaweeds that contain the light harvesting complex with photosynthetic pigments48. These results are explained by the absence of activity of the enzyme with ID 22 on the lipid and protein-rich plastid membrane, since this enzyme acts specifically on alginate and oligoalginates35.
Enzyme with ID 22 was able to release fatty acids from alga biomass to the extracellular medium (supernatant), although without a significant effect on the algal incubation residue. To date, only one study49 suggested the change of fatty acid profile promoted by alginate lyases on brown seaweeds, although using a alginate lyase from PL5 family acting on Undaria pinnatifida and with no statistical analysis of data. Thus, the present study is the first that show the significant effect of a recombinant alginate lyase (PL7) on fatty acid profile of a brown macroalga (L. digitata). It was observed an increase of total MUFA, such as 16:1c9, 18:1c9, 18:1c11 and 20:1c11 fatty acids, and a concomitant decrease of total SFA, including the major 16:0 and 18:0 fatty acids, released to the supernatant. In fact, oleic acid (18:1c9) was increased by threefold with the alginate lyase treatment. These results might be explained by the release of phlorotannins to the extraction medium by the action of alginate lyase, as previously reported11. In fact, tannins were previously shown to inhibit the complete biohydrogenation of C18 fatty acids in animals50. The latter aspects need to be further exploited due to the benefits that increasing the release of MUFA, such as 18:1c9, in detriment of SFA, have to human health, particularly on preventing cardiovascular diseases7.
Conclusion
The results obtained in the present study indicate that the sole use of an alginate lyase from PL7 family, under physiological conditions, can lead to a partial degradation of L. digitata cell wall. The disruption of macroalga cell wall would allow the release of trapped bioactive compounds with important value for biotechnological and feed industries. The high nutritional value of these compounds may stimulate the use of exogenous enzymes, as novel biocatalysts, to supplement diets containing L. digitata for monogastric animals. Further work is currently in progress in our research laboratories to assess the effectiveness of using this alginate lyase as a supplement for monogastric diets with high incorporation levels (10–15% of diet weight) of L. digitata.
Methods
Macroalga production
The low heat-dried brown macroalgae L. digitata was obtained from Algolesko Company (Plobannalec-Lesconil, Brittany, France), where it was cultivated in seawater offshore ponds and biologically certified by Ecocert. Before it was used for the in vitro incubations, the macroalgae was ground in a knife mill (Grindomix GM 200, Retsch Gmbh, Germany), sieved through a woven wire mesh sieve with a diameter of 63 µm (Retsch Gmbh, Germany) and stored at -20 ºC.
High-throughput gene synthesis, cloning and protein expression/purification of recombinant enzymes
One-hundred and seventy-six CAZymes with high potential for degradation of macroalgae cell wall were selected from a diverse repertoire, including glycoside hydrolases (GH), pectate lyases (PL) and carbohydrate esterases (CE). In addition, 23 sulfatases were selected for screening, as they are also likely involved in the degradation of sulphated polysaccharides from macroalgae cell walls51.
The generation of 199 recombinant plasmids, as well as the expression and purification of the corresponding enzymes, followed the procedures described in previous studies27,28. One-hundred and sixty-six coding genes for the selected enzymes were synthesised in vitro using NZYGene Synthesis kit (Nzytech, Portugal), whereas the other 33 coding genes were synthesised by Twist Bioscience (San Francisco, CA, USA). The sequence of each enzyme is presented in Supplementary Table S1. After optimisation of synthetic genes for cloning and subsequent expression in Escherichia coli, 166 genes were directly cloned into the bacterial expression vector pHTP1 (Nzytech, Portugal) using NZYEasy Cloning & Expression kit I (Nzytech, Portugal), whereas 33 genes were cloned in pET-29b( +) (Twist Bioscience, San Francisco, CA, USA). The generated recombinant plasmids were subjected to inducible T7 promoter control, while encoding the 199 enzymes fused to an N-terminal His6-tag that facilitates purification through Immobilised Metal Affinity Chromatography (IMAC). All recombinant plasmids were sequenced to ensure that no mutations accumulated during gene synthesis and were used to transform E. coli BL21 (DE3) cells in 24 deep-well plates, followed by protein production, cell harvesting and protein purification. An IMAC high-throughput method based on an automated protocol that allows the purification of 96 proteins simultaneously was performed, as previously reported52 but with some modifications. Briefly, 1 mL of crude cell lysates were incubated with 200 μL of 25% Ni2+ Sepharose 6 Fast flow resin (GE Healthcare, IL, USA) and transferred into 96-well filter plates (Macherey–Nagel, Duren, Germany)52. The wells were washed twice with 1 mL of 10 mM Imidazole buffer (10 mM Imidazole, 50 mM NaHepes, 1 M NaCl, 5 mM CaCl2, at pH = 7.5) and once with 1 mL of 35 mM Imidazole buffer (35 mM Imidazole, 50 mM NaHepes, 1 M NaCl, 5 mM CaCl2, at pH = 7.5). Then, the proteins were eluted with 300 µL of 300 mM Imidazole buffer (300 mM Imidazole, 50 mM NaHepes, 1 M NaCl, 5 mM CaCl2, at pH = 7.5). All steps of protein purification from cell-free extracts were automated on a Tecan robot (Tecan, Switzerland), containing a vacuum manifold. Homogeneity of purified proteins and molecular mass of recombinant enzymes were determined by SDS-PAGE in 14% (w/v) acrylamide gels and compared to a low molecular weight (LMW) protein marker (18.5 to 96 KDa) (Nzytech, Portugal). The gel images were acquired with BioRad ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA, USA). Protein concentration of enzyme stock solutions varied between 0.13–26.7 g/L, as determined spectrophotometrically on NanoDrop 2000/2000c (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA), and the level of protein expression was determined accordingly (see Supplementary Table S1).
Preparation of macroalga cell suspension
The preparation of L. digitata suspension at 20 g/L in PBS solution, including a pre-wash step, centrifugation and algae re-suspension, was done using the procedure previously described for microalgae27.
Enzymatic cell wall disruption
The cell wall disruption assay was performed in triplicate and with an enzyme concentration of 20 µg/ mL of incubation volume, as previously reported27, but with the following changes: the incubation of the 24 well microplate (VWR Chemicals, West Chester, PA, USA) containing macroalgae suspension and alginate lyase was performed overnight at 160 rpm. Then, the microplate was centrifuged for 30 min at 3210 g and the supernatants and pellets were recovered. To precipitate and remove the enzymes, the supernatant for DNSA and HPLC analyses was boiled for 5 min, centrifuged for 5 min at 10,000 g and the supernatant recovered.
Reducing sugars measurement
To quantify the released reducing sugars, the 3,5-dinitrosalicylic acid (DNSA) method53 was used as previously described for microalgae27, with the modification that 0.5 mL of glucose solutions or supernatants were mixed with 0.5 mL of DNSA reagent.
Fluorescence microscopic observations
The pellets from the enzyme cell wall disruption assay, were re-suspended in 0.5 mL of PBS solution for the initial screening or 1 mL of PBS for the assays with the most-active selected enzyme. The suspension was mixed by pipetting to ensure that the algae pellets were suspended evenly. The re-suspended macroalgae biomass concentration was 40 mg/mL. The fluorochrome Calcofluor White (Sigma-Aldrich, St. Louis, Mo, USA), that binds to the cell wall12,33,54, was added at 10 µL to the suspensions on adhesion slides (SuperFrost Plus Menzel Gläser, Thermo Scientific, Braunschweig, Germany), followed by a solution of 10% KOH (VWR Chemicals, West Chester, PA, USA), in a proportion of 1:1:1. The fluorescence microscopic procedures were done as previously reported27. Cells were observed with an epifluorescence microscope and images were captured with a Leica DFC-340FX (Leica, Wetzlar, Germany) camera system, in order to determine the fluorescence intensity, expressed as arbitrary units, by using the Image J software (NIH, Bethesda, MA, USA).
Determination of catalytic activity of alginate lyase
The catalytic activity of alginate lyase was analysed by two different methods: UV spectroscopy and determination of reducing sugars. The UV spectroscopy analysis followed the procedures described in a previous report55, with some modifications. Briefly, a mixture of 1 mL of PBS solution containing 1% NaCl (pH = 7.5), 0.5 mL of alginic acid from brown algae (Sigma-Aldrich, Darmstadt, Germany) and 5 µl of alginate lyase (ID 22) at 4.37 mg/mL were mixed in a quartz cuvette. The alginate was previously dissolved in a PBS solution at a concentration of 0.3%. The increase in absorbance at λmax 233 nm was continuously recorded during 1 h in an UV/Vis Spectrophotometer (Pharmacia LKB Ultrospec III spectrophotometer, Gemini, Apeldoorn, Netherlands), at 37 °C, to verify linearity. The enzyme activity was maximum during 2 min. The maximum activity was reported in absorbance units (AU) defined as the increase in absorbance units per minute. The determination of reducing sugars was done after stopping the enzyme reaction by using the DNSA reagent with subsequent heating of the samples, following the procedures previously described for the measurement of reducing sugars27.
Thermostability and proteolysis experiments
The alginate lyase was biochemically characterized, specifically for thermostability and proteolysis resistance. The recombinant enzyme was purified through IMAC using gravity flow columns (His GraviTrap™, GE Healthcare, IL, USA), according to a previously described procedure29,30. The protein concentration was adjusted at 0.83 g/L for both assays. The thermostability analysis was performed as previously reported27. The protein concentration in the recovered supernatant was quantified in triplicate using a NanoDrop 2000/2000c (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA), and the results were validated through visualization of 14% SDS-PAGE gels, showing the intensity of the bands present in the supernatants. The gel images were acquired with BioRad ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA, USA). The proteolysis resistance analysis was performed as already described27. The alginate lyase was incubated with porcine pancreatin (VWR Chemicals, West Chester, PA, USA) or PBS solution and, afterwards, the samples were analysed by 14% SDS-PAGE and compared to a low molecular weight (LMW) protein marker (18.5 to 96 KDa) (Nzytech, Portugal) (Fig. 5). The resultant images were acquired with BioRad ChemiDoc XRS imaging system (Bio-Rad) and proteolysis was confirmed by visualizing fragments with different molecular weights.
Determination of mono- and oligosaccharides
The profile of mono- and oligosaccharides from the supernatants derived from incubation of L. digitata with control and alginate assays was analysed and quantified by high performance liquid chromatography (HPLC). For the analysis of monosaccharide profile, the chromatogram data was compared with individual monosaccharides standards composed by arabinose, galactose, glucose, mannose plus xylose. Additional monosaccharides, including ribose, fructose, rhamnose, fucose, as well as its derivatives, such as galactosamine, glucosamine, sorbitol and glucoronic acid were also used for comparison. For the analysis of oligosaccharide profile, the chromatogram data was compared with a mixture of cellulose-derived oligosaccharides. The procedures followed a previously developed protocol27. The quantification of total oligosaccharides was based on a standard curve, using a range of concentrations from 0.025 to 0.60 mM of glucose. The results were expressed as equivalent moles of released glucose per 100 g of macroalga.
Determination of protein content
After L. digitata suspension and incubation with control and alginate assays, the N content in lyophilised supernatant and residue fractions was quantified by the Kjeldahl method (984.13)56, assuming that no nitrogen from the media interfere with the assay. The crude protein was calculated as N × 4.9257.
Pigment analysis
The content of chlorophyll a, chlorophyll b, total carotenoids and pheophytins were quantified in supernatant and residue fractions from L. digitata suspension, after control and alginate assays, as described by Hynstova et al.58, with slight modifications previously reported27, except that total carotenoids also included the amount of fucoxanthins. The fucoxanthin content was quantified in the same way as the other pigments, but using the following formula described in a recent study59:
Cfuc = 6.39 × A445 − 5.18 × A663, where Cfuc is the concentration of fucoxanthin (mg/ml), A445 is the absorbance at λmax 445 nm and A663 is the absorbance at λmax 663 nm.
Determination of fatty acid composition
Fatty acids from the lyophilised supernatants and pellets of L. digitata after control and alginate assays were extracted as already described for microalgae27. Fatty acids were esterified to methyl esters (FAME) by acidic catalysis based on the procedure described in a previous report4, but using 5 ml of acetylchloride-methanol solution (1.25 M Sigma-Aldrich, St. Louis, Mo, USA) for up to 24.3 mg of sample. The analysis of FAME was done following procedures previously reported27, except for the quantification of total FAME, that was carried out using nonadecanoic acid (19:0) as internal standard. Each fatty acid was expressed as a percentage of the sum of identified fatty acids (% total fatty acids). The fatty acid present in a percentage inferior to 0.5% were included as others in Table 3.
Statistical analysis
Data were analysed using the Generalised Linear Mixed (GLM) model of the SAS software package (version 9.4; SAS Institute Inc., Cary, NC, USA), except data from the thermostability experiment, which were analysed using the MIXED procedure of SAS. Normality was checked using Shapiro–Wilk test. All experiments were conducted in triplicate, except for the initial screening where experiments were conducted in duplicate. The error bars on figures indicate the standard error of the mean (SEM). Results are presented as mean and SEM, and were considered significantly different when p < 0.05.
Data availability
All data generated during this study are included in this published article. The datasets generated during the current study are available from the corresponding author on demand.
References
Makkar, H. P. S. et al. Seaweeds for livestock diets: A review. Anim. Feed Sci. Tech. 212, 1–17. https://doi.org/10.1016/j.anifeedsci.2015.09.018 (2016).
Holdt, S. L. & Kraan, S. Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol. 23, 543–597. https://doi.org/10.1007/s10811-010-9632-5 (2011).
Costa, M., Cardoso, C., Afonso, C., Bandarra, N. M. & Prates, J. A. M. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: a systematic review. J. Anim. Physiol. Anim. Nutr. 00, 1–28. https://doi.org/10.1111/jpn.13509 (2021).
Cardoso, C. et al. Fatty acid profiles of the main lipid classes of green seaweeds from fish pond aquaculture. Food Sci. Nutr. 5, 1186–1194. https://doi.org/10.1002/fsn3.511 (2017).
Neto, R. T. et al. Screening of Ulva rigida, Gracilaria sp, Fucus vesiculosus and Saccharina latissima as functional ingredients. Int. J. Mol. Sci. https://doi.org/10.3390/Ijms19102987 (2018).
Campos, A. M. et al. Azorean macroalgae (Petalonia binghamiae, Halopteris scoparia and Osmundea pinnatifida) bioprospection: a study of fatty acid profiles and bioactivity. Int. J. Food Sci. Technol. 54, 880–890. https://doi.org/10.1111/ijfs.14010 (2019).
Givens, I. Animal nutrition and lipids in animal products and their contribution to human intake and health. Nutrients 1, 71–82. https://doi.org/10.3390/nu1010071 (2009).
FAO. World review in The State of World Fisheries and Aquaculture. Meeting the sustainable development goals (ed. FAO) 25, 74 (FAO, 2018).
Popper, Z. A. et al. Evolution and diversity of plant cell walls: from algae to flowering plants. Annu. Rev. Plant Biol. 62, 567–590. https://doi.org/10.1146/annurev-arplant-042110-103809 (2011).
Michel, G., Tonon, T., Scornet, D., Cock, J. M. & Kloareg, B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol. 188, 82–97. https://doi.org/10.1111/j.1469-8137.2010.03374.x (2010).
Deniaud-Bouet, E. et al. Chemical and enzymatic fractionation of cell walls from Fucales: insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 114, 1203–1216. https://doi.org/10.1093/aob/mcu096 (2014).
Salmeán, A. A. et al. Insoluble (1 -> 3), (1 -> 4)-β-D-glucan is a component of cell walls in brown algae (Phaeophyceae) and is masked by alginates in tissues. Sci. Rep. 7, 2880. https://doi.org/10.1038/S41598-017-03081-5 (2017).
Deniaud-Bouet, E., Hardouin, K., Potin, P., Kloareg, B. & Herve, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 175, 395–408. https://doi.org/10.1016/j.carbpol.2017.07.082 (2017).
Kadam, S. U., Tiwari, B. K. & O’Donnell, C. P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 50, 24–31. https://doi.org/10.1111/ijfs.12692 (2015).
Dobrincic, A. et al. Advanced technologies for the extraction of marine brown algal polysaccharides. Mar. Drugs https://doi.org/10.3390/Md18030168 (2020).
Øverland, M., Mydland, L. T. & Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agr. 99, 13–24. https://doi.org/10.1002/jsfa.9143 (2019).
Adams, J. M., Gallagher, J. A. & Donnison, I. S. Fermentation study on Saccharina latissima for bioethanol production considering variable pre-treatments. J. Appl. Phycol. 21, 569–574. https://doi.org/10.1007/s10811-008-9384-7 (2009).
Batista, S. et al. Use of technological processing of seaweed and microalgae as strategy to improve their apparent digestibility coefficients in European seabass (Dicentrarchus labrax) juveniles. J. Appl. Phycol. 32, 3429–3446. https://doi.org/10.1007/s10811-020-02185-2 (2020).
Fleurence, J., Massiani, L., Guyader, O. & Mabeau, S. Use of enzymatic cell-wall degradation for improvement of protein extraction from Chondrus crispus, Gracilaria verrucosa and Palmaria palmata. J. Appl. Phycol. 7, 393–397. https://doi.org/10.1007/Bf00003796 (1995).
Harnedy, P. A. & FitzGerald, R. J. Extraction of protein from the macroalga Palmaria palmata. Food Sci. Technol. 51, 375–382. https://doi.org/10.1016/j.lwt.2012.09.023 (2013).
Joubert, Y. & Fleurence, J. Simultaneous extraction of proteins and DNA by an enzymatic treatment of the cell wall of Palmaria palmata (Rhodophyta). J. Appl. Phycol. 20, 55–61. https://doi.org/10.1007/s10811-007-9180-9 (2008).
Mæhre, H. K., Jensen, I. J. & Eilertsen, K. E. Enzymatic pre-treatment increases the protein bioaccessibility and extractability in Dulse (Palmaria palmata). Mar. Drugs https://doi.org/10.3390/Md14110196 (2016).
Hou, X. R., Hansen, J. H. & Bjerre, A. B. Integrated bioethanol and protein production from brown seaweed Laminaria digitata. Bioresour. Technol. 197, 310–317. https://doi.org/10.1016/j.biortech.2015.08.091 (2015).
Ravanal, M. C. et al. The role of alginate lyases in the enzymatic saccharification of brown macroalgae, Macrocystis pyrifera and Saccharina latissima. Algal Res. 26, 287–293. https://doi.org/10.1016/j.algal.2017.08.012 (2017).
Vanegas, C. H., Hernon, A. & Bartlett, J. Enzymatic and organic acid pretreatment of seaweed: effect on reducing sugars production and on biogas inhibition. Int. J. Ambient Energy 36, 2–7. https://doi.org/10.1080/01430750.2013.820143 (2015).
Habeebullah, S. F. K. et al. Enzyme-assisted extraction of bioactive compounds from brown seaweeds and characterization. J. Appl. Phycol. 32, 615–629. https://doi.org/10.1007/s10811-019-01906-6 (2020).
Coelho, D. et al. Novel combination of feed enzymes to improve the degradation of Chlorella vulgaris recalcitrant cell wall. Sci. Rep. 9, 5382. https://doi.org/10.1038/S41598-019-41775-0 (2019).
Coelho, D. et al. A two-enzyme constituted mixture to improve the degradation of Arthrospira platensis microalga cell wall for monogastric diets. J. Anim. Physiol. Anim. Nutr. 104, 310–321. https://doi.org/10.1111/jpn.13239 (2020).
Costa, M. et al. Construction of GH16 β-glucanase mini-cellulosomes to improve the nutritive value of barley-based diets for broilers. J. Agr. Food Chem. 62, 7496–7506. https://doi.org/10.1021/jf502157y (2014).
Fernandes, V. O. et al. 1,3–1,4-β-glucanases and not 1,4-β-glucanases improve the nutritive value of barley-based diets for broilers. Anim. Feed Sci. Technol. 211, 153–163. https://doi.org/10.1016/j.anifeedsci.2015.11.007 (2016).
Ravindran, V. & Son, J. H. Feed enzyme technology: present status and future developments. Recent Pat. Food Nutr. Agric. 3, 102–109. https://doi.org/10.2174/2212798411103020102 (2011).
Sichert, A. et al. Verrucomicrobia use hundreds of enzymes to digest the algal polysaccharide fucoidan. Nat. Microbiol. 5, 1026–1039. https://doi.org/10.1038/s41564-020-0720-2 (2020).
Holzinger, A., Herburger, K., Kaplan, F. & Lewis, L. A. Desiccation tolerance in the chlorophyte green alga Ulva compressa: does cell wall architecture contribute to ecological success?. Planta 242, 477–492. https://doi.org/10.1007/s00425-015-2292-6 (2015).
Zverlov, V., Volkov, I., Velikodvorskaya, T. & Schwarz, W. Highly thermostable endo-1,3-β-glucanase (laminarinase) LamA from Thermotoga neapolitana: Nucleotide sequence of the gene and characterization of the recombinant gene product. Microbiology 143, 1701–1708. https://doi.org/10.1099/00221287-143-5-1701 (1997).
Kim, H. T. et al. Characterization of a recombinant endo-type alginate lyase (Alg7D) from Saccharophagus degradans. Biotechnol. Lett. 34, 1087–1092. https://doi.org/10.1007/s10529-012-0876-9 (2012).
Rodríguez-Diaz, J., Carbajo, R., Pineda-Lucena, A., Monedero, V. & Yebra, M. Synthesis of fucosyl-N-acetylglucosamine disaccharides by transfucosylation using α-L-fucosidases from Lactobacillus casei. Appl. Environ Microbiol. 79, 3847–3850. https://doi.org/10.1128/AEM.00229-13 (2013).
Fontes, C. M. G. A., Hall, J., Hirst, B. H., Hazlewood, G. P. & Gilbert, H. J. The resistance of cellulases and xylanases to proteolytic inactivation. Appl. Microbiol. Biotechnol. 43, 52–57. https://doi.org/10.1007/BF00170622 (1995).
Ekborg, N. A. et al. Saccharophagus degradans gen. nov., sp. nov., a versatile marine degrader of complex polysaccharides. Int. J. Syst. Evol. Microbiol. 55, 1545–1549. https://doi.org/10.1099/ijs.0.63627-0 (2005).
Mishima, Y. et al. A super-channel in bacteria: macromolecule uptake and depolymerization systems of Sphingomonas sp A1 with a special cell surface structure. Biotechnol. Genet. Eng. Rev. 19, 105–119. https://doi.org/10.1080/02648725.2002.10648025 (2002).
Wang, D. M. et al. Optimal production of 4-deoxy-L-erythro-5-hexoseulose uronic acid from alginate for brown macro algae saccharification by combining endo- and exo-type alginate lyases. Bioprocess. Biosyst. Eng. 37, 2105–2111. https://doi.org/10.1007/s00449-014-1188-3 (2014).
Manns, D., Deutschle, A. L., Saake, B. & Meyer, A. S. Methodology for quantitative determination of the carbohydrate composition of brown seaweeds (Laminariaceae). Rsc Adv. 4, 25736–25746. https://doi.org/10.1039/c4ra03537b (2014).
Pilgaard, B. et al. Proteomic enzyme analysis of the marine fungus Paradendryphiella salina reveals alginate lyase as a minimal adaptation strategy for brown algae degradation. Sci. Rep. https://doi.org/10.1038/S41598-019-48823-9 (2019).
Loomis, W. D. & Battaile, J. Plant phenolic compounds and the isolation of plant enzymes. Phytochemistry 5, 423–438. https://doi.org/10.1016/S0031-9422(00)82157-3 (1966).
Jordan, P. & Vilter, H. Extraction of proteins from material rich in anionic mucilages: Partition and fractionation of vanadate-dependent bromoperoxidases from the brown algae Laminaria digitata and L saccharina in aqueous polymer two-phase systems. Biochim. Biophys. Acta 1073, 98–106. https://doi.org/10.1016/0304-4165(91)90188-M (1991).
Fleurence, J. The enzymatic degradation of algal cell walls: a useful approach for improving protein accessibility?. J. Appl. Phycol. 11, 313–314. https://doi.org/10.1023/A:1008183704389 (1999).
Osório, C. et al. Pigments content (chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations 7, 33. https://doi.org/10.3390/separations7020033 (2020).
Kanazawa, K. et al. Commercial-scale preparation of biofunctional fucoxanthin from waste parts of brown sea algae Laminaria japonica. Food Sci. Technol. Res. 14, 573–582. https://doi.org/10.3136/Fstr.14.573 (2008).
Lichtlé, C., Spilar, A. & Duval, J. Immunogold localization of light-harvesting and photosystem I complexes in the thylakoids of Fucus serratus (Phaeophyceae). Protoplasma 166, 99–106 (1992).
Billakanti, J. M., Catchpole, O. J., Fenton, T. A., Mitchell, K. A. & MacKenzie, A. D. Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process Biochem. 48, 1999–2008. https://doi.org/10.1016/j.procbio.2013.09.015 (2013).
Lee, M. R. F., Tweed, J. K. S., Cookson, A. & Sullivan, M. L. Immunogold labelling to localize polyphenol oxidase (PPO) during wilting of red clover leaf tissue and the effect of removing cellular matrices on PPO protection of glycerol-based lipid in the rumen. J. Sci. Food Agric. 90, 503–510. https://doi.org/10.1002/jsfa.3848 (2010).
Helbert, W. Marine polysaccharide sulfatases. Front. Mar. Sci. https://doi.org/10.3389/fmars.2017.00006 (2017).
Saez, N. J. & Vincentelli, R. High-throughput expression screening and purification of recombinant proteins in E. coli. Methods Mol. Biol. 1091, 33–53. https://doi.org/10.1007/978-1-62703-691-7_3 (2014).
Miller, G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428. https://doi.org/10.1021/Ac60147a030 (1959).
Schoenwaelder, M. E. A. & Clayton, M. N. The presence of phenolic compounds in isolated cell walls of brown algae. Phycologia 38, 161–166. https://doi.org/10.2216/i0031-8884-38-3-161.1 (1999).
Østgaard, K. Enzymatic microassay for the determination and characterization of alginates. Carbohydr. Polym. 19, 51–59. https://doi.org/10.1016/0144-8617(92)90054-T (1992).
AOAC. Official methods of analysis, 17th ed (Association of Official Analytical Chemists, 2000).
Lourenço, S. O., Barbarino, E., De-Paula, J. C., Pereira, L. O. D. S. & Marquez, U. M. L. Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycological Res. 50, 233–241. https://doi.org/10.1046/j.1440-1835.2002.00278.x (2002).
Hynstova, V. et al. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using high performance thin layer chromatography. J. Pharm. Biomed. Anal. 148, 108–118. https://doi.org/10.1016/j.jpba.2017.09.018 (2018).
Wang, L. J. et al. A rapid method for the determination of fucoxanthin in diatom. Mar. Drugs 16, 33. https://doi.org/10.3390/Md16010033 (2018).
Acknowledgments
This study was supported by Fundação para a Ciência e a Tecnologia (FCT, Lisbon, Portugal; grant PTDC/CAL-ZOO/30238/2017), and CIISA (Project UIDB/00276/2020) and a PhD fellowship to DC (SFRH/BD/126198/2016).
Author information
Authors and Affiliations
Contributions
M.C. prepared macroalgae biomass for the subsequent experiments. P.B. and C.M.G.A.F. constructed the databank with CAZYmes and sulfatases. M.C. and P.B. generated recombinant plasmids. V.C. and J.B. were responsible for high-throughput production of recombinant enzymes. M.C. and L.P. preformed the incubations and analysed reducing sugars and fluorescence intensity of algal cell walls, as well as pigment and protein contents. M.C. and C.M.A. analysed fat composition of macroalgae samples, and C.M.A. analysed oligosaccharides amount. M.C. and D.C. performed statistics. D.C. assisted in the execution of the experiments. M.C. performed the manuscript preparation and literature review. J.A.M.P. contributed to the overall research design, interpretation and discussion of the experimental results. All authors have revised, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Costa, M., Pio, L., Bule, P. et al. An individual alginate lyase is effective in the disruption of Laminaria digitata recalcitrant cell wall. Sci Rep 11, 9706 (2021). https://doi.org/10.1038/s41598-021-89278-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89278-1
- Springer Nature Limited
This article is cited by
-
High-Voltage Pulsed Electric Fields and pH Shift Process for Protein and Solute Release from Gracilaria sp., Red Edible Seaweed
Food and Bioprocess Technology (2024)
-
High voltage pulsed electric field and electroporation technologies for algal biomass processing
Journal of Applied Phycology (2024)
-
Effect of Laminaria digitata dietary inclusion and CAZyme supplementation on blood cells, serum metabolites and hepatic lipids and minerals of weaned piglets
Scientific Reports (2023)
-
Combined effects of dietary Laminaria digitata with alginate lyase on plasma metabolites and hepatic lipid, pigment and mineral composition of broilers
BMC Veterinary Research (2022)