Abstract
In some species of social insects the increased genetic diversity from having multiple breeders in a colony has been shown to improve pathogen resistance. Termite species typically found colonies from single mated pairs and therefore may lack the flexibility to buffer pathogen pressure with increased genetic diversity by varying the initial number of reproductives. However, they can later increase group diversity through colony merging, resulting in a genetically diverse, yet cohesive, workforce. In this study, we investigate whether the increased group diversity from colony fusion benefits social immunity in the subterranean termite Reticulitermes flavipes. We confirm previous findings that colonies of R. flavipes will readily merge and we show that workers will equally groom nestmates and non-nestmates after merging. Despite this, the survival of these merged colonies was not improved after exposure to a fungal pathogen, but instead leveled to that of the more susceptible or the more resistant colony. Our study brings little support to the hypothesis that colony fusion may improve immunity through an increase of genetic diversity in R. flavipes. Instead, we find that following exposure to a lethal pathogen, one colony is heavily influential to the entire group’s survival after merging.
Similar content being viewed by others
Introduction
Social insects are among the most abundant and ecologically successful species1. Their success is inextricably linked to their division of labor where workers engage in different tasks to benefit one or a few reproductives at the expense of their own reproduction. The low number of reproductives in colonies of most species results in high relatedness among nestmate workers, elevating indirect fitness benefits2. Paradoxically, their social life also entails severe costs, as high worker densities, high relatedness, and closed nests strongly increase the chance of pathogen transmission, which would suggest that these species are vulnerable to disease outbreaks3. Owing to such pathogenic pressure, social insects have evolved social immunity, whereby individual immune functions and behaviors collectively provide colony-wide disease protection4,5,6. Social immunity includes self/allogrooming7,8,9,10,11,12,13,14, nest hygiene15,16,17,18,19,20,21,22, removal of diseased individuals23,24,25,26 and the use of antimicrobial compounds either produced by individuals or from materials incorporated into the nest27,28,29,30,31,32,33,34,35,36,37,38. These diverse immune strategies have undoubtedly reduced the costs of social living, facilitating the success of social insects.
Colony resistance to pathogens is also associated with within-colony genetic diversity, as genetically distinct individuals may vary in their susceptibility to different disease strains39,40,41,42,43,44,45. Therefore, a mix of distinct genotypes within a colony interferes with genotype x genotype interactions, such that a pathogen able to infect one genotype may fail to transmit to new hosts if it encounters host genotypes that it cannot infect. Consequently, a genetically diverse colony may reduce the overall spread of a pathogen. Although variation in susceptibility may increase the likelihood of an infection, it may also prevent the risk that an outbreak of a single strain of pathogen wipes out all individuals, as a diverse colony will only lose a fraction of its population41. In addition, the efficiency of social immunity may increase with within-colony genetic diversity whereby genetically distinct individuals differ in their propensities to detect, survive and respond to different pathogens. Therefore, genetically diverse colonies may be better protected against the threat of a variety of disease agents3,41,46,47.
In social Hymenoptera, colonies may increase their level of genetic diversity by the presence of several reproductive queens (i.e., polygyny) and queens mated with several males (i.e., polyandry)48,49. Despite these strategies being associated with a reduction of relatedness within colonies (i.e., the indirect fitness of workers), they have evolved several times in social Hymenoptera50. One of the main hypotheses suggests that the increased genetic diversity that results from the high number of reproductives in a colony can strengthen their resistance toward pathogens3,39,46. Empirical studies have shown improved pathogen resistance from polyandry in bumblebees51,52,53, honeybees40,42,43,44,54,55,56,57, and ants58, as well as from polygyny in ants59. The beneficial effect of genetic diversity on social immunity has been widely examined in social Hymenoptera, but its evidence in termites, the other major group of social insects, is scarce.
Termite colonies share several features with Hymenopteran colonies even though sociality evolved independently in this group60,61. However, they differ from social Hymenoptera in that their colonies are founded by a single pair of primary reproductives, the queen and the king. Consequently, as termites have a relatedness initially locked at 0.5, they lack the flexibility to potentially buffer variable pathogen pressure with increased genetic diversity by varying the initial number of reproductives. After the founding stage, though, many termite species are able to merge colonies, which can result in a genetically diverse, yet cohesive, workforce62. The factors underlying colony merging are not well understood. Discrimination between nestmates and non-nestmates may play an important role in colony merging. In the subterranean termite, Reticulitermes flavipes, merged colonies had strong similarities in their mitochondrial DNA. The close relatedness between merging colonies, associated with a shared maternally inherited factor, may decrease nestmate recognition and favor colony fusion63. Potentially, nestmate recognition could also be related to the gut microbiota, as the microbial communities of termite guts are colony-specific64. In the species R. speratus, colonies can be made to accept foreign workers by experimentally altering the microbial gut communities65. In this species, colonies are also more likely to merge if the introduced colony has low proportions of nymphs66. Termite nymphs have high resource demands67, which makes them energetically expensive for the receiving colony. Thus, colony merging may be influenced by the current status of the colonies involved. The costs and benefits of taking in additional, unrelated workers likely vary across different colonies and species. It is also possible that colony merging could not be a specific behavior that is selected for, but a byproduct of distinct colonies expanding their foraging galleries in the same food source. As they consume more wood and expand their foraging galleries, then those colonies would not be able to remain separate.
Colony merging occurs naturally in R. flavipes68,69,70,71,72 and this species shows a lack of intercolonial aggression in laboratory assays73,74. In this study, we investigated whether the increased group diversity through colony merging benefits social immunity and pathogen resistance in R. flavipes. We first confirmed the ability of R. flavipes to fuse colonies by investigating merging rate and aggression between pairs of colonies through a behavioral assay. Potentially, merged colonies could preferentially groom their relatives, so we also assessed whether social immunity was symmetrical in artificially merged colonies by comparing the amount of grooming between nestmates and non-nestmates. Finally, we tested the hypothesis that increased group diversity enhances pathogen resistance by determining whether the survival of artificially merged colonies was higher than that of single colonies against an entomopathogenic fungus. Further, we determined if single colonies benefited from specific pairings by comparing the survival of each merged colony pairing to that of its two constituent colonies. Finally, we discuss the ecological and evolutionary factors underlying termite colony merging from the perspective of social immunity.
Results
Colony merging
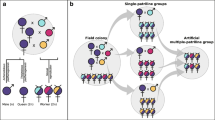
Intercolonial aggression and colony merging was determined using a behavioral assay designed to test agonism between groups of termites75. We recorded aggressive behaviors exhibited between termites from a colony of R. flavipes that were either paired with termites from another R. flavipes colony, a group of termites from the same colony, or termites from a colony of the related species, R. virginicus. Aggressive behavior was strongly associated with the type of pairing that was tested (χ2 = 38.973, p < 0.0005; Fig. 1a). Out of the 28 pairings between groups of R. flavipes originating from two different colonies, 27 merged without any evidence of aggression. Only one pairing (colonies E and H) resulted in aggression. In the positive control, no aggression was found in pairings between two groups of the same colony. In the negative control, all pairings between colonies of R. flavipes and R. virginicus displayed aggressive behaviors, showing the ability of R. flavipes to exhibit agonistic behaviors while in this experimental setting.
(a) The proportion of behaviors observed in each type of pairing in the agonism assay. The asterisks indicates that aggressive behavior was highly associated with interspecific pairings between R. flavipes and R. virginicus (χ2-test, p < 0.0005). (b) The pathogen assay arena with examples of mutual grooming between dyed and undyed termites, originally from different colonies. (c) Colonies exposed to a pathogen treatment spent more time grooming (Nested ANOVA, p < 0.05), but there was no difference in grooming between single (N = 6) and merged colonies (N = 6) (Nested ANOVA, p = 0.8849). (d) There was no difference in the time that termites from merged colonies spent grooming nestmates or non-nestmates (Nested ANOVA, p = 0.90711, N = 6). All analyses were performed in the statistical software R 3.5.0 (https://www.r-project.org/).
Grooming behavior
We measured grooming in groups of termite workers that either all originated from the same colony or were artificially merged from two different colonies. In these merged groups, one colony was previously fed a blue dye, so that both colonies could be distinguished from each other (Fig. 1b). These single and merged colony groups were exposed to either a control solution or a solution containing conidia of the fungal pathogen, Metarhizium robertsii, at a concentration of 1×106 conidia/mL. Then, we recorded the time spent grooming, as well as whether the grooming was directed towards nestmates or non-nestmates. We observed a significant increase in grooming within termite groups exposed to a pathogen, in both single and artificially merged colonies (p < 0.05; Fig. 1c). However, the total duration of grooming did not differ significantly between single and merged colonies, in both control and pathogen groups (p = 0.8849; Fig. 1c). In merged colonies, termites did not invest significantly more time in grooming nestmates or non-nestmates, whether they were exposed to a control or pathogen solution (p = 0.90711; Fig. 1d).
Survival of single and merged colonies
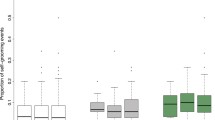
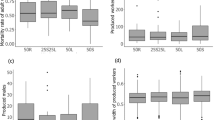
Colonies exposed to the pathogen treatment had 20–25% lower survival (p < 0.001; Fig. 2). We also found that the survival of merged colonies exposed to a pathogen was slightly lower than the survival of single colonies (p < 0.05; Fig. 2). When we examined each specific merged colony pairing, we found that in five out of the six pairing combinations, the two single colonies used to build the artificially merged colony had significantly different survivals. In four of these pairings, the merged colony survival aligned with the survival of the more susceptible of the two single colonies (Fig. 3a–d). In the fifth pairing, the merged colony survival matched the more resistant of the two single colonies (Fig. 3e). In the last pairing, where the survival distributions of the two single colonies were not different from each other, the survival of the merged colony did not differ from either of the two single colonies (Fig. 3f). The results of the pairwise comparisons, including those made between controls are provided in Supplementary Information T1.
Kaplan-Meier survival distributions of all single (N = 6) and merged colony (N = 6) groups that were exposed to either a control or pathogen solution. Termites exposed to a pathogen had significantly lower survival than termites which received a control solution (p < 0.001). The asterisk indicates that merged colonies were found to have slightly lower survival than single colonies (p < 0.05). All analyses were performed in the statistical software R 3.5.0 (https://www.r-project.org/).
Kaplan-Meier survival distributions of each merged colony pair (Red, N = 1) are plotted with the survival distributions of their corresponding single colonies (Light and dark blue, N = 2). Bolded letters (a–f) correspond to each of the six groupings of merged and single colonies that were tested. Within each plot, letters denote significant groupings between pathogen treatment groups. The survival distributions of control groups are depicted in the plots, but are not included in groupings. Significance was determined by pairwise comparisons using a log-rank test (p < 0.05; Supplementary Information T1). All analyses were performed in the statistical software R 3.5.0 (https://www.r-project.org/).
Discussion
We confirmed that colonies of this species readily merge in the lab and showed that workers groom nestmates and non-nestmates equally after merging. These two results are prerequisites for colony merging to test for improved resistance to pathogens. However, the survival of merged colonies was not improved from that of single colonies when challenged with a pathogen. Instead, our results showed that the overall survival of each merged colony was heavily influenced by the survival of the colonies from which it was composed. In most cases, the survival of the merged colony was reduced to that of the more susceptible colony, but in one case the survival of the group was raised to that of the more resistant colony. Our study brings little support to the hypothesis that colony merging may improve immunity through an increase of genetic diversity in R. flavipes.
In R. flavipes, different colonies have been shown to vary considerably in their ability to resist different strains of pathogens45. This finding was supported by our study, as most of the single colonies differed in their survival against the entomopathogenic fungus studied (Fig. 3). However, most studies, including ours, used a single generalist pathogenic agent to challenge colonies. The use of a diverse pathogen cocktail may further test the hypothesis that increased diversity within a colony will provide protection against a broad range of pathogen pressures41, while it also better represents natural pressures that colonies encounter. In addition, it would be interesting to test whether the use of generalist or specialist pathogen agents have distinct outcomes on colony survival. Termites can display a vibratory alarm to their nestmates in response to pathogens76,77. In R. flavipes the strength of this alarm varies between colonies and is positively correlated with the time that nestmates spend grooming, which in turn predicts their survival78. Thus, variation in survival may also be influenced by the variation among colonies to detect, and therefore respond or avoid different pathogens. In our study, we ensured the exposure of individuals to the pathogen by flooding the substrate with a pathogen suspension in a small arena. This setup allows the infection of all termites to measure differential survival, but hampers their survival through avoidance. We also showed for the first time that workers equally groom nestmates and non-nestmates after merging (Fig. 1d). One would therefore expect merged colonies to survive at the level of the more resistant colony, as workers with greater detection ability should be able to groom the entire merged colony. However, our finding that survival is lower in merged colonies indicates that the more susceptible colony may determine the level of group susceptibility.
Several mechanisms of social immunity have been examined in termites. Nest structures are typically constructed from fecal material, which can inhibit the growth of harmful microbes79. Termite nests have also been found to harbor beneficial Actinobacteria, which are known to possess antimicrobial activity35,38. Colonies can maintain hygienic conditions by cannibalizing nestmates that are infected or have recently succumbed to disease25,80,81,82. Additionally, the ‘social transfer’ of disease resistance has been reported in the dampwood termite species, Zootermopsis angusticollis, where individuals have improved survival against a fungal pathogen after being grouped with individuals that have survived a challenge with the same pathogen83. However, grooming is one of the most effective mechanisms of social immunity that has been studied in termites. Workers kept in isolation have much greater mortality than those kept in groups after exposure to a fungal pathogen10,13,84. Termite salivary glands produce antimicrobial peptides which, when applied though grooming, effectively inhibit the growth of fungal pathogens85,86,87. In addition, grooming allows termites to remove fungal spores attached to the cuticle of a nestmate. These spores are swallowed and ultimately end up in the gut of their nestmates, where they are unable to germinate13,88. Even once the conidia have penetrated the cuticle, and the infected termite can no longer be saved, nestmates still show a large grooming response25. This intense grooming may also ultimately lead to cannibalism to prevent dying individuals from proliferating disease. The unyielding nature of termite grooming behavior may help provide constant protection from disease spreading throughout the colony.
Termite colonies undergo developmental changes throughout their lifespan, which may affect the role that genetic diversity plays in immunity. In many termite species, including R. flavipes, genetic diversity may decline over time due to the development of secondary reproductives in the colony (i.e., transition to an extended-family)62,89. When the founding king and queen perish, the workers or nymphs of the colony may become secondary kings and queens that engage in repeated inbreeding over time. These secondary reproductives prolong the life of the colony at the expense of reduced genetic diversity within the colony. Thus, extended-family colonies may be more likely to merge in order to restore the level of genetic diversity within colonies. Indeed, naturally merged colonies of R. flavipes appear to merge shortly after the death of the founders of one of the constituent colonies90. Genetic diversity may not only be important to older colonies that experience inbreeding, but also to those that are still young. In R. flavipes and R. virginicus, the proportion of mature colonies headed by inbred reproductives is lower than the proportion of inbred pairs found during the nuptial flight suggesting that inbreeding depression negatively affects developing colonies91. Colony foundation represents an important threshold for termite survival, as young colonies undergo strong selective pressure92. In Z. angusticollis, inbreeding between founding kings and queens produces offspring with increased disease susceptibility in comparison to outbred mating93. It has also been shown that the effects of pathogen exposure during early colony foundation constrain the reproductive output and overall survival of the future colony in this species94. The king and queen play pivotal roles in incipient colonies. Until the workforce is large enough to completely sustain the colony, the king and queen are responsible for protecting themselves and rearing the first brood. Thus, the survival of incipient colonies is largely dependent on the fitness of the founding pair94,95,96.
In social Hymenoptera, strong support for improved disease resistance from increased genetic diversity has been demonstrated in bumblebees51,52,53 and honeybees40,42,43,44,54,55,56,57, while similar experiments in ants have produced contrasting results. While several ant species show no relationship between genetic diversity and pathogen resistance97,98,99, there are some that do. In the leaf-cutting ant, Acromrymex echinatior, workers from different patrilines vary in their susceptibility to a fungal pathogen, suggesting that a genetically diverse worker force may increase the overall colony survival against a variety of pathogens58. In Formica selysi, naturally polygynous colonies do not have higher survival than monogynous colonies, but artificially combined workers from different monogynous colonies do show improved disease resistance59. While there is some indication that genetic diversity could provide health benefits for both ants and termites, strong evidence is still lacking for its relevance in natural settings.
In R. flavipes colonies vary in their ability to detect and respond to disease, they will readily merge, and workers will groom each other equally in merged colonies. Despite this, we do not find evidence that genetic diversity improves pathogen resistance in R. flavipes. Instead we observed that after merging, one colony heavily influenced the survival of the group. The factors that determine which colony is more influential to immunity after merging remain unknown. Additionally, there are some caveats to this study that may warrant further investigation. While our method of testing merging rates among colony fragments is sufficient to ensure that our merged colonies would not attack each other during the experiment, termites may still maintain colony boundaries without aggression. A study examining whether colony fragments of R. flavipes would share foraging and nesting sites reported merging rates to be as low as 55% in laboratory assays74. In our study, we found balanced grooming within the merged colonies, but the interactions between colonies that have fused may be more complex. Potentially, by allowing mixed colonies to acclimate together, the two colonies may have developed a combined colony odor that may preclude any kin recognition in grooming. As with what has been found in ants, the relationship between genetic diversity and immunity in termites may vary among species. Further studies investigating the proximate mechanisms by which changes in the genetic architecture of merged colonies, such as an increase in allelic diversity, heterozygosity, or differential gene expression, would affect colony survival are clearly worth more attention. Investigations into the potential benefits of colony merging in this and other species are needed to determine whether fitness gains derived from increased genetic diversity drive colony merging in termites.
Methods
Termite and pathogen collection
We collected groups of termites from 20 colonies of R. flavipes and one colony of the related species R. virginicus. The colonies were sampled from wood debris found in College Station, Texas from October 2018 to February 2019. Based on previous studies, all collections were made at least 15 m apart to ensure that each colony was unique90,100,101. In R. flavipes, mate pairing is random during large, synchronous nuptial flights, leading to an absence of isolation-by-distance patterns at short distances, meaning that two geographically close colonies are not genetically more similar than two geographically distant colonies69,100,102,103. As a consequence, our sampled colonies likely represent a spectrum of varying levels of relatedness, regardless of their sampling distances. The termites were separated from wood in the lab, assigned an identifying letter (A-T) and maintained in rearing chambers at 85% relative humidity and 27 °C. All termites were used in experiments within 2 weeks of collection. The DNA of one worker per colony was extracted and sequenced at the 16 S mitochondrial locus to confirm the species identification of the colonies sampled (Supplementary Information S1).
A field-collected strain of the fungus Metarhizium robertsii was used in the pathogenic treatment for this study. This strain was isolated from soil collected from the Sam Houston National Forest, TX using a mealworm baiting method, then cultured on a medium of potato dextrose agar104. The identity of the strain was confirmed through sequencing analysis of the ITS region (Supplementary Information S1). Fungal conidia were collected from the medium and suspended in a 0.1% TWEEN80 solution. This solution was concentrated at 1 × 106 conidia/mL using a hemocytometer (Bulldog Bio, Inc. Portsmouth, NH, USA) and used as a pathogen treatment. The 0.1% TWEEN80 solution without fungal conidia was used as a control. These pathogen and control solutions were used for all immune challenges in this study.
Colony merging
We performed an agonism assay using the arena described in Chouvenc & Su 2017. Ten workers and one soldier from two different colonies were introduced into opposite sides of a 3×10 cm arena and were allowed to tunnel through sand along a preformed path, until the two groups encountered each other75. We tested every combination of eight different R. flavipes colonies (colonies A-H; N = 28) and recorded any signs of aggression between colonies (biting, tunnel blocking, casualties, etc.). As controls, we also tested all eight of these colonies against themselves (N = 8), as well as against the colony of R. virginicus (N = 8). All pairings were monitored for behavior every 15 minutes for 3 hours after introduction, which was enough time for termites from both sides to connect the tunnels and interact with each other. Pairings were denoted as aggressive if any signs of aggression between the two groups was observed. The two groups were considered to have merged if there were no signs of aggression and workers freely moved between both sides of the assay.
Grooming behavior
Six colonies (colonies I-N) were used to determine the amount of grooming in the presence of pathogens, as well as to compare the amount of grooming between nestmates and non-nestmates within artificially merged colonies. At the time of collection, a subset of workers from each colony were fed cellulose material containing Nile blue (a fat-soluble stain used to mark termites) so that workers from different colonies could be identified when mixed with another colony (Fig. 1b). Nile blue has been used in a number of studies to mark termites and has not been reported to affect termite behavior25,83,105,106. Groups of 20 workers were set up with either 20 workers from the same colony (i.e., single colony), or by combining 10 dyed workers from one colony with 10 undyed workers from another colony to create six merged colonies (JxK, JxL, KxL, MxN, MxO and NxO; bolded letters indicate the dyed colony). Groups were isolated in 60 mm petri dishes with moist filter paper and allowed to acclimate for 1 week before exposure. Two identical replicates were performed for all single colony (N = 6) and merged colony (N = 6) groups. Pathogen and control solutions were applied by pipetting 200 μL of solution onto the filter paper in each petri dish. Five-minute videos were recorded for each petri dish 15 minutes after the solution application. The time spent grooming other termites was recorded for every individual and then totaled for each replicate. In merged colonies, the direction of grooming (towards nestmates or non-nestmates) was also recorded for each sub-colony within a merged colony (N = 6).
Survival of single and merged colonies
From the remaining six colonies (colonies O-T), groups of 20 workers were set up with either 20 workers from the same colony (i.e., single colony), or by combining 10 workers each from two different colonies into the same dish (i.e., merged colony; PxQ, PxR, QxR, SxT, SxU and TxU). Groups were isolated in 60 mm petri dishes with moist filter paper and allowed to acclimate for 1 week before exposure. All of these combinations (N = 6) were simultaneously replicated four times with the same pathogen and control solutions. Treatments were applied by pipetting 200 μL of solution onto the filter paper in each petri dish. Mortality was recorded daily for 2 weeks following the solution application.
Statistical analysis
A Pearson’s χ2 test of independence was used to determine if aggressive behavior was associated with pairings between species, within species, or within colonies. Grooming time was compared between single and merged colonies using a nested ANOVA, with colony status (single colony or merged colony) nested within treatment group. We also used a nested ANOVA to determine if grooming in merged colonies was directed more towards nestmates or non-nestmates, with grooming direction nested within treatment group. Using the coxph function implemented in the survival package in R107, termite mortality in the survival assay was analyzed using a Cox proportional hazard survival model with the factor of colony status nested within treatment group. Pairwise comparisons using a log-rank test were performed for the survival distributions of every merged colony and its two corresponding single colonies. To avoid inflation of Type I errors, the Benjamini-Hochberg procedure for adjusting p-values was used. All analyses were performed in the statistical software R 3.5.0108.
Data availability
The data reported in this study have been deposited in the Open Science Framework database, https://osf.io (https://doi.org/10.17605/OSF.IO/B73RZ).
References
Wilson, E. O. Success and dominance in ecosystems: the case of the social insects in Excellence in Ecology Vol. 2 (ed. Kinne, O.) (Ecology Institute, 1990).
Hamilton, W. D. The genetic theory of social behavior. I and II. Journal of Theoretical Biology 7, 1–52 (1964).
Hamilton, W. Kinship, recognition, disease, and intelligence: constraints of social evolution in Animal Societies: Theories and Facts (eds. Ito, Y., Brown, J. L., & Kikkawa, J.) 81–100 (Japan Scientific Societies Press, 1987).
Cremer, S., Armitage, S. A. & Schmid-Hempel, P. Social immunity. Current Biology 17, R693–R702 (2007).
Cremer, S., Pull, C. D. & Fuerst, M. A. Social immunity: emergence and evolution of colony-level disease protection. Annual Review of Entomology 63, 105–123 (2018).
Liu, L., Zhao, X.-Y., Tang, Q.-B., Lei, C.-L. & Huang, Q.-Y. The mechanisms of social immunity against fungal infections in eusocial insects. Toxins 11, 244 (2019).
Peng, Y.-S., Fang, Y., Xu, S. & Ge, L. The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. Journal of Invertebrate Pathology 49, 54–60 (1987).
Drees, B. M., Miller, R. W., Vinson, B. S. & Georgis, R. Susceptibility and behavioral response of red imported fire ant (Hymenoptera: Formicidae) to selected entomogenous nematodes (Rhabditida: Steinernematidae & Heterorhabditidae). Journal of Economic Entomology 85, 365–370 (1992).
Oi, D. H. & Pereira, R. M. Ant behavior and microbial pathogens (Hymenoptera: Formicidae). Florida Entomologist 76, 63–74 (1993).
Rosengaus, R. B., Maxmen, A. B., Coates, L. E. & Traniello, J. F. Disease resistance: a benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera: Termopsidae). Behavioral Ecology and Sociobiology 44, 125–134 (1998).
Hughes, W. O., Eilenberg, J. & Boomsma, J. J. Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proceedings of the Royal Society of London. Series B: Biological Sciences 269, 1811–1819 (2002).
Wilson-Rich, N., Stuart, R. J. & Rosengaus, R. B. Susceptibility and behavioral responses of the dampwood termite Zootermopsis angusticollis to the entomopathogenic nematode Steinernema carpocapsae. Journal of Invertebrate Pathology 95, 17–25 (2007).
Yanagawa, A. & Shimizu, S. Resistance of the termite, Coptotermes formosanus Shiraki to Metarhizium anisopliae due to grooming. BioControl 52, 75–85 (2007).
Liu, L. et al. The influence of allogrooming behavior on individual innate immunity in the subterranean termite Reticulitermes chinensis (Isoptera: Rhinotermitidae). Journal of Insect Science 19, 6 (2019).
Howard, D. F. & Tschinkel, W. R. Aspects of necrophoric behavior in the red imported fire ant, Solenopsis invicta. Behaviour 56, 157–178 (1976).
Siebeneicher, S. R., Bradleigh, S. & Kenerley, C. M. Infection of the red imported fire ant by Beauveria bassiana through various routes of exposure. Journal of Invertebrate Pathology 59, 280–285 (1992).
Trumbo, S. T., Huang, Z.-Y. & Robinson, G. E. Division of labor between undertaker specialists and other middle-aged workers in honey bee colonies. Behavioral Ecology and Sociobiology 41, 151–163 (1997).
Julian, G. E. & Cahan, S. Undertaking specialization in the desert leaf-cutter ant Acromyrmex versicolor. Animal Behaviour 58, 437–442 (1999).
Bot, A. N., Currie, C. R., Hart, A. G. & Boomsma, J. J. Waste management in leaf-cutting ants. Ethology Ecology & Evolution 13, 225–237 (2001).
Hart, A. G. & Ratnieks, F. L. Waste management in the leaf-cutting ant Atta colombica. Behavioral Ecology 13, 224–231 (2002).
Ballari, S., Farji-Brener, A. G. & Tadey, M. Waste management in the leaf-cutting ant Acromyrmex lobicornis: division of labour, aggressive behaviour, and location of external refuse dumps. Journal of Insect Behavior 20, 87–98 (2007).
Sun, Q. & Zhou, X. Corpse management in social insects. International Journal of Biological Sciences 9, 313–321 (2013).
Heinze, J. & Walter, B. Moribund ants leave their nests to die in social isolation. Current Biology 20, 249–252 (2010).
Rueppell, O., Hayworth, M. & Ross, N. Altruistic self-removal of health-compromised honey bee workers from their hive. Journal of Evolutionary Biology 23, 1538–1546 (2010).
Davis, H. E., Meconcelli, S., Radek, R. & McMahon, D. P. Termites shape their collective behavioural response based on stage of infection. Scientific Reports 8, 14433 (2018).
Pull, C. D. et al. Destructive disinfection of infected brood prevents systemic disease spread in ant colonies. eLife 7, e32073 (2018).
Brown, W. L. Jr. An hypothesis concerning the function of the metapleural glands in ants. The American Naturalist 102, 188–191 (1968).
Hölldobler, B. & Engel-Siegel, H. On the metapleural gland of ants. Psyche: A Journal of Entomology 91, 201–224 (1984).
Gilliam, M., Taber, S. III, Lorenz, B. J. & Prest, D. B. Factors affecting development of chalkbrood disease in colonies of honey bees, Apis mellifera, fed pollen contaminated with Ascosphaera apis. Journal of Invertebrate Pathology 52, 314–325 (1988).
Ortius-Lechner, D., Maile, R., Morgan, E. D. & Boomsma, J. J. Metapleural gland secretion of the leaf-cutter ant Acromyrmex octospinosus: new compounds and their functional significance. Journal of Chemical Ecology 26, 1667–1683 (2000).
Christe, P., Oppliger, A., Bancalà, F., Castella, G. & Chapuisat, M. Evidence for collective medication in ants. Ecology Letters 6, 19–22 (2003).
Turillazzi, S. et al. Dominulin A and B: two new antibacterial peptides identified on the cuticle and in the venom of the social paper wasp Polistes dominulus using MALDI-TOF, MALDI-TOF/TOF, and ESI-ion trap. Journal of the American Society for Mass Spectrometry 17, 376–383 (2006).
Chapuisat, M., Oppliger, A., Magliano, P. & Christe, P. Wood ants use resin to protect themselves against pathogens. Proceedings of the Royal Society of London. Series B: Biological Sciences 274, 2013–2017 (2007).
Simone, M., Evans, J. D. & Spivak, M. Resin collection and social immunity in honey bees. Evolution: International Journal of Organic Evolution 63, 3016–3022 (2009).
Visser, A. A., Nobre, T., Currie, C. R., Aanen, D. K. & Poulsen, M. Exploring the potential for Actinobacteria as defensive symbionts in fungus-growing termites. Microbial Ecology 63, 975–985 (2012).
Rosengaus, R. B., Mead, K., Du Comb, W. S., Benson, R. W. & Godoy, V. G. Nest sanitation through defecation: antifungal properties of wood cockroach feces. Naturwissenschaften 100, 1051–1059 (2013).
Arango, R. et al. Antimicrobial activity of actinobacteria isolated from the guts of subterranean termites. Environmental Entomology 45, 1415–1423 (2016).
Chouvenc, T., Elliott, M. L., Šobotník, J., Efstathion, C. A. & Su, N.-Y. The termite fecal nest: a framework for the opportunistic acquisition of beneficial soil Streptomyces (Actinomycetales: Streptomycetaceae). Environmental Entomology 47, 1431–1439 (2018).
Shykoff, J. A. & Schmid-Hempel, P. Parasites and the advantage of genetic variability within social insect colonies. Proceedings of the Royal Society of London. Series B: Biological Sciences 243, 55–58 (1991).
Palmer, K. A. & Oldroyd, B. P. Evidence for intra-colonial genetic variance in resistance to American foulbrood of honey bees (Apis mellifera): further support for the parasite/pathogen hypothesis for the evolution of polyandry. Naturwissenschaften 90, 265–268 (2003).
van Baalen, M. & Beekman, M. The costs and benefits of genetic heterogeneity in resistance against parasites in social insects. The American Naturalist 167, 568–577 (2006).
Bourgeois, A. L., Rinderer, T. E., Sylvester, H. A., Holloway, B. & Oldroyd, B. P. Patterns of Apis mellifera infestation by Nosema ceranae support the parasite hypothesis for the evolution of extreme polyandry in eusocial insects. Apidologie 43, 539–548 (2012).
Evison, S. E. et al. Host–parasite genotypic interactions in the honey bee: the dynamics of diversity. Ecology and Evolution 3, 2214–2222 (2013).
Lee, G., McGee, P. & Oldroyd, B. Variable virulence among isolates of Ascosphaera apis: testing the parasite–pathogen hypothesis for the evolution of polyandry in social insects. Naturwissenschaften 100, 229–234 (2013).
Denier, D. & Bulmer, M. Variation in subterranean termite susceptibility to fatal infections by local Metarhizium soil isolates. Insectes Sociaux 62, 219–226 (2015).
Sherman, P. W., Seeley, T. D. & Reeve, H. K. Parasites, pathogens, and polyandry in social Hymenoptera. The American Naturalist 131, 602–610 (1988).
Schmid-Hempel, P. Parasites in Social Insects. (Princeton University Press, 1998).
Bourke, A. F. & Franks, N. R. Social Evolution in Ants. (Princeton University Press, 1995).
Crozier, R. H. & Fjerdingstad, E. J. Polyandry in social Hymenoptera—disunity in diversity? Annales Zoologici Fennici 38, 267–285 (2001).
Hughes, W., Ratnieks, F. & Oldroyd, B. Multiple paternity or multiple queens: two routes to greater intracolonial genetic diversity in the eusocial Hymenoptera. Journal of Evolutionary Biology 21, 1090–1095 (2008).
Liersch, S. & Schmid-Hempel, P. Genetic variation within social insect colonies reduces parasite load. Proceedings of the Royal Society of London. Series B: Biological Sciences 265, 221–225 (1998).
Baer, B. & Schmid-Hempel, P. Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature 397, 151–154 (1999).
Baer, B. & Schmid-Hempel, P. Unexpected consequences of polyandry for parasitism and fitness in the bumblebee, Bombus terrestris. Evolution 55, 1639–1643 (2001).
Tarpy, D. R. Genetic diversity within honeybee colonies prevents severe infections and promotes colony growth. Proceedings of the Royal Society of London. Series B: Biological Sciences 270, 99–103 (2003).
Seeley, T. D. & Tarpy, D. R. Queen promiscuity lowers disease within honeybee colonies. Proceedings of the Royal Society of London. Series B: Biological Sciences 274, 67–72 (2006).
Tarpy, D. R. & Seeley, T. D. Lower disease infections in honeybee (Apis mellifera) colonies headed by polyandrous vs monandrous queens. Naturwissenschaften 93, 195–199 (2006).
Mattila, H. R., Rios, D., Walker-Sperling, V. E., Roeselers, G. & Newton, I. L. Characterization of the active microbiotas associated with honey bees reveals healthier and broader communities when colonies are genetically diverse. PLoS One 7, e32962 (2012).
Hughes, W. O. & Boomsma, J. J. Genetic diversity and disease resistance in leaf-cutting ant societies. Evolution 58, 1251–1260 (2004).
Reber, A., Castella, G., Christe, P. & Chapuisat, M. Experimentally increased group diversity improves disease resistance in an ant species. Ecology Letters 11, 682–689 (2008).
Thorne, B. L. Evolution of eusociality in termites. Annual Review of Ecology and Systematics 28, 27–54 (1997).
Nalepa, C. A. Origin of termite eusociality: trophallaxis integrates the social, nutritional, and microbial environments. Ecological Entomology 40, 323–335 (2015).
Vargo, E. L. Diversity of termite breeding systems. Insects 10, 52 (2019).
DeHeer, C. & Vargo, E. Strong mitochondrial DNA similarity but low relatedness at microsatellite loci among families within fused colonies of the termite Reticulitermes flavipes. Insectes Sociaux 55, 190–199 (2008).
Minkley, N., Fujita, A., Brune, A. & Kirchner, W. Nest specificity of the bacterial community in termite guts (Hodotermes mossambicus). Insectes Sociaux 53, 339–344 (2006).
Matsuura, K. Nestmate recognition mediated by intestinal bacteria in a termite, Reticulitermes speratus. Oikos 92, 20–26 (2001).
Matsuura, K. & Nishida, T. Colony fusion in a termite: What makes the society “open”? Insectes Sociaux 48, 378–383 (2001).
Thorne, B. L. Alate production and sex ratio in colonies of the Neotropical termite Nasutitermes corniger (Isoptera; Termitidae). Oecologia 58, 103–109 (1983).
Jenkins, T. M., Basten, C. J., Kresovich, S. & Forshcler, B. Mitochondrial gene sequence questions Reticulitermes sp. social structure (Isoptera: Rhinotermitidae). Sociobiology 34, 161–172 (1999).
Bulmer, M. S., Adams, E. S. & Traniello, J. F. Variation in colony structure in the subterranean termite Reticulitermes flavipes. Behavioral Ecology and Sociobiology 49, 236–243 (2001).
Deheer, C. J. & Kamble, S. T. Colony genetic organization, fusion and inbreeding in Reticulitermes flavipes from the Midwestern US. Sociobiology 51, 307–325 (2008).
Perdereau, E., Bagnères, A.-G., Dupont, S. & Dedeine, F. High occurrence of colony fusion in a European population of the American termite Reticulitermes flavipes. Insectes Sociaux 57, 393–402 (2010).
Majid, A. A., Kamble, S. T. & Chen, H. Breeding patterns and population genetics of eastern subterranean termites Reticulitermes flavipes in urban environment of Nebraska, United States. Sociobiology 65, 506–514 (2018).
Polizzi, J. M. & Forschler, B. T. Factors that affect aggression among the worker caste of Reticulitermes spp. subterranean termites (Isoptera: Rhinotermitidae). Journal of Insect Behavior 12, 133–146 (1999).
Fisher, M. L., Gold, R. E., Vargo, E. L. & Cognato, A. I. Behavioral and genetic analysis of colony fusion in Reticulitermes flavipes (Isoptera: Rhinotermitidae). Sociobiology 44, 565–576 (2004).
Chouvenc, T. & Su, N.-Y. Testing the role of cuticular hydrocarbons on intercolonial agonism in two subterranean termite species (Coptotermes) and their hybrids. Insectes Sociaux 64, 347–355 (2017).
Rosengaus, R. B., Jordan, C., Lefebvre, M. L. & Traniello, J. F. A. Pathogen alarm behavior in a termite: a new form of communication in social insects. Naturwissenschaften 86, 544–548 (1999).
Myles, T. G. Alarm, aggregation, and defense by Reticulitermes flavipes in response to a naturally occurring isolate of Metarhizium anisopliae. Sociobiology 40, 243–256 (2002).
Bulmer, M. S., Franco, B. A. & Fields, E. G. Subterranean termite social alarm and hygienic responses to fungal pathogens. Insects 10, 240 (2019).
Rosengaus, R. B., Guldin, M. R. & Traniello, J. F. Inhibitory effect of termite fecal pellets on fungal spore germination. Journal of Chemical Ecology 24, 1697–1706 (1998).
Rosengaus, R. B. & Traniello, J. F. Disease susceptibility and the adaptive nature of colony demography in the dampwood termite Zootermopsis angusticollis. Behavioral Ecology and Sociobiology 50, 546–556 (2001).
Chouvenc, T. & Su, N.-Y. When subterranean termites challenge the rules of fungal epizootics. PLoS One 7, e34484 (2012).
Sun, Q., Haynes, K. F. & Zhou, X. Differential undertaking response of a lower termite to congeneric and conspecific corpses. Scientific Reports 3, 1650 (2013).
Traniello, J. F., Rosengaus, R. B. & Savoie, K. The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proceedings of the National Academy of Sciences 99, 6838–6842 (2002).
Shimizu, S. & Yamaji, M. Effect of density of the termite, Reticulitermes speratus Kolbe (Isoptera: Rhinotermitidae), on the susceptibilities to Metarhizium anisopliae. Applied Entomology and Zoology 38, 125–130 (2003).
Lamberty, M. et al. Insect immunity constitutive expression of a cysteine-rich antifungal and a linear antibacterial peptide in a termite insect. Journal of Biological Chemistry 276, 4085–4092 (2001).
Bulmer, M. S. & Crozier, R. H. Duplication and diversifying selection among termite antifungal peptides. Molecular Biology and Evolution 21, 2256–2264 (2004).
Hamilton, C. & Bulmer, M. S. Molecular antifungal defenses in subterranean termites: RNA interference reveals in vivo roles of termicins and GNBPs against a naturally encountered pathogen. Developmental & Comparative Immunology 36, 372–377 (2012).
Chouvenc, T., Su, N.-Y. & Robert, A. Inhibition of Metarhizium anisopliae in the alimentary tract of the eastern subterranean termite Reticulitermes flavipes. Journal of Invertebrate Pathology 101, 130–136 (2009).
Myles, T. G. Review of secondary reproduction in termites (Insecta: Isoptera) with comments on its role in termite ecology and social evolution. Sociobiology 33, 1–43 (1999).
Deheer, C. J. & Vargo, E. L. Colony genetic organization and colony fusion in the termite Reticulitermes flavipes as revealed by foraging patterns over time and space. Molecular Ecology 13, 431–441 (2004).
DeHeer, C. J. & Vargo, E. L. An indirect test of inbreeding depression in the termites Reticulitermes flavipes and Reticulitermes virginicus. Behavioral Ecology and Sociobiology 59, 753–761 (2006).
Nutting, W. Flight and colony foundation in Biology of Termites Vol. 1 (eds. Krishna, K. & Weesner, F. M.) 233–282 (Academic Press, 1969).
Calleri, D. V., McGrail Reid, E., Rosengaus, R. B., Vargo, E. L. & Traniello, J. F. Inbreeding and disease resistance in a social insect: effects of heterozygosity on immunocompetence in the termite Zootermopsis angusticollis. Proceedings of the Royal Society of London. Series B: Biological Sciences 273, 2633–2640 (2006).
Cole, E. L., Ilieş, I. & Rosengaus, R. B. Competing physiological demands during incipient colony foundation in a social insect: consequences of pathogenic stress. Frontiers in Ecology and Evolution 6, 103 (2018).
Matsuura, K. & Nishida, T. Comparison of colony foundation success between sexual pairs and female asexual units in the termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Population Ecology 43, 119–124 (2001).
Matsuura, K., Fujimoto, M., Goka, K. & Nishida, T. Cooperative colony foundation by termite female pairs: altruism for survivorship in incipient colonies. Animal Behaviour 64, 167–173 (2002).
Briano, J., Patierson, R. & Cordo, H. Relationship between colony size of Solenopsis richteri (Hymenoptera: Formicidae) and infection with Thelohania solenopsae (Microsporida: Thelohaniidae) in Argentina. Journal of Economic Entomology 88, 1233–1237 (1995).
Pérez-Lachaud, G., Valenzuela, J. E. & Lachaud, J.-P. Is increased resistance to parasitism at the origin of polygyny in a Mexican population of the ant Ectatomma tuberculatum (Hymenoptera: Formicidae)? Florida Entomologist 94, 677–684 (2011).
Schmidt, A. M., Linksvayer, T. A., Boomsma, J. J. & Pedersen, J. S. No benefit in diversity? The effect of genetic variation on survival and disease resistance in a polygynous social insect. Ecological Entomology 36, 751–759 (2011).
Vargo, E. L. Hierarchical analysis of colony and population genetic structure of the eastern subterranean termite, Reticulitermes flavipes, using two classes of molecular markers. Evolution 57, 2805–2818 (2003).
DeHeer, C., Kutnik, M., Vargo, E. & Bagneres, A. The breeding system and population structure of the termite Reticulitermes grassei in Southwestern France. Heredity 95, 408–415 (2005).
Vargo, E. L. & Carlson, J. R. Comparative study of breeding systems of sympatric subterranean termites (Reticulitermes flavipes and R. hageni) in central North Carolina using two classes of molecular genetic markers. Environmental Entomology 35, 173–187 (2006).
Vargo, E. L., Juba, T. R. & Deheer, C. J. Relative abundance and comparative breeding structure of subterranean termite colonies (Reticulitermes flavipes, Reticulitermes hageni, Reticulitermes virginicus, and Coptotermes formosanus) in a South Carolina lowcountry site as revealed by molecular markers. Annals of the Entomological Society of America 99, 1101–1109 (2006).
Hughes, W. O., Thomsen, L., Eilenberg, J. & Boomsma, J. J. Diversity of entomopathogenic fungi near leaf-cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. Journal of Invertebrate Pathology 85, 46–53 (2004).
Yanagawa, A. et al. Behavioral changes in the termite, Coptotermes formosanus (Isoptera), inoculated with six fungal isolates. Journal of Invertebrate Pathology 107, 100–106 (2011).
Chouvenc, T., Su, N.-Y. & Elliott, M. L. Interaction between the subterranean termite Reticulitermes flavipes (Isoptera: Rhinotermitidae) and the entomopathogenic fungus Metarhizium anisopliae in foraging arenas. Journal of Economic Entomology 101, 885–893 (2008).
Therneau, T. M. & Lumley, T. Package ‘survival’. R Top Doc 128 (2015).
R. Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org (2018).
Acknowledgements
We thank Alan Byboth and the Sam Houston State University Center for Biological Field Studies for access to soil samples that were used to cultivate fungal pathogens. We also thank Alex Blumenfeld and Dylan Sjølie for their help in collecting termites, as well as Johnathan Koehl for his help in screening for effective pathogens. This research was supported by the Urban Entomology Endowment at Texas A&M University.
Author information
Authors and Affiliations
Contributions
C.M.A., P.A.E. and E.L.V. designed the study. C.M.A. and P.A.E. collected data. C.M.A. analyzed the data. C.M.A., P.A.E., and E.L.V. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aguero, C.M., Eyer, PA. & Vargo, E.L. Increased genetic diversity from colony merging in termites does not improve survival against a fungal pathogen. Sci Rep 10, 4212 (2020). https://doi.org/10.1038/s41598-020-61278-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61278-7
- Springer Nature Limited
This article is cited by
-
Defensive behavior is linked to altered surface chemistry following infection in a termite society
Scientific Reports (2023)
-
Genetic diversity, paternal origin and pathogen resistance in Cataglyphis desert ants
Behavioral Ecology and Sociobiology (2023)
-
Comparative genetic study of the colony structure and colony spatial distribution between the higher termite Amitermes parvulus and the lower, subterranean termite Reticulitermes flavipes in an urban environment
Insectes Sociaux (2023)
-
Short and long-term costs of inbreeding in the lifelong-partnership in a termite
Communications Biology (2022)
-
Distinct chemical blends produced by different reproductive castes in the subterranean termite Reticulitermes flavipes
Scientific Reports (2021)