Abstract
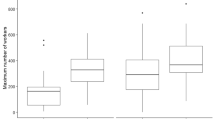
Group genetic diversity is usually associated with a reduced risk of disease outbreak and a slower rate of pathogen transmission. In social insects, multiple mating by queens (polyandry) evolved several times albeit reducing worker’s inclusive fitness. One major hypothesis suggests that polyandry has been selected for to mitigate the risk of outbreak thanks to increased genetic diversity within colonies. We investigated this hypothesis in the ant Cataglyphis mauritanica, in which nestmate workers are produced by several clonal, single-mated queens. Using natural colonies, we correlated genetic diversity with worker survival to a fungal entomopathogen. We further tested whether workers from different paternal lineages (but a common maternal genome) show differential resistance in experimentally single- or multiple-patriline groups, and whether an increased number of patrilines in a group improved disease resistance. We show that workers from distinct patrilines vary in their resistance to a pathogen in single-patriline colonies, but the difference among patrilines disappears when they are mixed in multiple-patriline colonies. Furthermore, pathogen resistance was affected by the number of patrilines in a group, with two- and three-patriline groups being more resistant than single-patriline groups. However, resistance did not differ between groups made of two and three patrilines; similarly, it was not associated with genetic diversity in natural colonies. Overall, our results suggest that collective disease defenses might homogenize workers’ resistance from different patrilines and, thereby, stabilize colony resistance.
Significance Statement
The occurrence of multiple breeders in insect societies has been hypothesized to be selected for because increased within-colony genetic diversity reduces the risk of severe outbreaks. We show that nestmate workers from distinct paternal lineages vary in their resistance to pathogens when reared in single-patriline groups. However, this difference disappears when workers are mixed in multiple-patriline groups. These results suggest that multiple mating by queens dilutes the deleterious consequences of a single patriline producing only susceptible offspring, rather than directly enhancing pathogen resistance.





Similar content being viewed by others
Data availability
Data generated in this study have been made publicly available on the Open Science Framework depository(https://osf.io/4tu5r/). https://doi.org/10.17605/OSF.IO/4TU5R
References
Aguero CM, Eyer PA, Vargo EL (2020) Increased genetic diversity from colony merging in termites does not improve survival against a fungal pathogen. Sci Rep 10:4212
Aguero CM, Eyer PA, Crippen TL, Vargo EL (2021a) Reduced environmental microbial diversity on the cuticle and in the galleries of a subterranean termite compared to surrounding soil. Microb Ecol 81:1054–1063
Aguero CM, Eyer PA, Martin JS, Bulmer MS, Vargo EL (2021b) Natural variation in colony inbreeding does not influence susceptibility to a fungal pathogen in a termite. Ecol Evol 11:3072–3083
Altizer S, Harvell D, Friedle E (2003) Rapid evolutionary dynamics and disease threats to biodiversity. Trends Ecol Evol 18:589–596
Anderson RM, May RM (1981) The population dynamics of microparasites and their invertebrate hosts. Philos Trans R Soc Lond B 291:451–524
Anderson RM, May RM (1986) The invasion, persistence and spread of infectious diseases within animal and plant communities. Philos Trans R Soc Lond B 314:533–570
Armitage SAO, Boomsma JJ (2010) The effects of age and social interactions on innate immunity in a leaf-cutting ant. J Insect Physiol 56:780–787
Armitage SAO, Broch JF, Marín HF, Nash DR, Boomsma JJ (2011) Immune defense in leaf-cutting ants: a cross-fostering approach. Evolution 65:1791–1799
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Aron S, Darras H, Eyer PA, Leniaud L, Pearcy M (2013) Structure génétique des sociétés et systèmes d’accouplement chez la fourmi Cataglyphis viatica (Fabricius 1787). Bull Inst Sci Rabat 35:103–109
Aron S, Mardulyn P, Leniaud L (2016) Evolution of reproductive traits in Cataglyphis desert ants: mating frequency, queen number, and thelytoky. Behav Ecol Sociobiol 70:1367–1379
Baer B, Schmid-Hempel P (1999) Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature 397:151–154
Baer B, Schmid-Hempel P (2001) Unexpected consequences of polyandry for parasitism and fitness in the bumblebee, Bombus terrestris. Evolution 55:1639–1643
Baer B, Schmid-Hempel P (2003) Bumblebee workers from different sire groups vary in susceptibility to parasite infection. Ecol Let 6:106–110
Bocher A, Tirard C, Doums C (2007) Phenotypic plasticity of immune defence linked with foraging activity in the ant Cataglyphis velox. J Evol Biol 20:2228–2234
Boomsma JJ, Ratnieks FLW (1996) Paternity in eusocial Hymenoptera. Philos Trans R Soc B Biol Sci 351:947–975
Boomsma JJ, Schmid-Hempel P, Hughes WOH (2005) Life histories and parasite pressure across the major groups of social insects. In: Fellowes M, Holloway G, Rolff G (eds) Insect Evolutionary Ecology. CABI, Wallingford, pp 139–175
Boomsma JJ, Kronauer DJC, Pedersen JS (2009) The evolution of social insect mating system. Harvard University Press, Cambridge, p 611
Boomsma JJ, Huszár DB, Pedersen JS (2014) The evolution of multiqueen breeding in eusocial lineages with permanent physically differentiated castes. Anim Behav 92:241–252
Boulay R, Arnan X, Cerdá X, Retana J (2014) The ecological benefits of larger colony size may promote polygyny in ants. J Evol Biol 27:2856–2863
Bourgeois AL, Rinderer T, Sylvester HA, Holloway B, Oldroyd B (2012) Patterns of Apis mellifera infestation by Nosema ceranae support the parasite hypothesis for the evolution of extreme polyandry in eusocial insects. Apidologie 43:539–548
Bourke AFG, Franks NR (1995) Social evolution in ants. Princeton University Press, Princeton, p 550
Brahma A, Leon RG, Hernandez GL, Wurm Y (2022) Larger, more connected societies of ants have a higher prevalence of viruses. Mol Ecol 31:859–865
Briano JA, Patterson RS, Cordo HA (1995) Relationship between colony size of Solenopsis richteri (Hymenoptera: Formicidae) and infection with Thelohania solenopsae (Microsporida : Thelohaniidae) in Argentina. J Eco Entomol 88:1233–1237
Bull JC, Ryabov EV, Prince G, Mead A, Zhang C et al (2012) A strong immune response in young adult honeybees masks their increased susceptibility to infection compared to older bees. PLoS Pathogen 8:e1003083
Calleri DV, McGrail RE, Rosengaus RB, Vargo EL, Traniello JF (2006) Inbreeding and disease resistance in a social insect: effects of heterozygosity on immunocompetence in the termite Zootermopsis angusticollis. Proc R Soc b: Biol Sci 273:2633–2640
Castella G, Christe P, Chapuisat M (2010) Covariation between colony social structure and immune defences of workers in the ant Formica selysi. Insect Soc 57:233–238
Chouvenc T, Su NY, Robert A (2009) Cellular encapsulation in the eastern subterranean termite, Reticulitermes flavipes (Isoptera), against infection by the entomopathogenic fungus Metarhizium anisopliae. J Invert Pathol 101:234–241
Chouvenc T, Bardunias P, Efstathion CA, Chakrabarti S, Elliott ML, Giblin-Davis R, Su NY (2013) Resource opportunities from the nest of dying subterranean termite (Isoptera: Rhinotermitidae) colonies: a laboratory case of ecological succession. Ann Entomol Soc Am 106:771–777
Crawford KM, Crutsinger GM, Sanders NJ (2007) Genotypic diversity mediates the distribution of an ecosystem engineer. Ecology 88:2114–2120
Cremer S, Sixt M (2009) Analogies in the evolution of individual and social immunity. Proc R Soc b: Biol Sci 364:129–142
Cremer S, Armitage SAO, Schmid-Hempel P (2007) Social immunity. Curr Biol 17:693–702
Cremer S, Pull CD, Fuerst MA (2018) Social immunity: Emergence and evolution of colony-level disease protection. Ann Rev Entomol 63:105–123
Crozier RH, Fjerdingstad EJ (2001) Polyandry in social Hymenoptera - disunity in diversity? Ann Zool Fenn 38:267–285
de Souza DJ, Van Vlaenderen J, Moret Y, Lenoir A (2008) Immune response affects ant trophallactic behaviour. J Insect Physiol 54:828–832
Desai SD, Currie RW (2015) Genetic diversity within honey bee colonies affects pathogen load and relative virus levels in honey bees, Apis mellifera L. Behav Ecol Sociobiol 69:1527–1541
Dwyer G, Elkington JS (1993) Using simple models to predict virus epizootics in gypsy moth populations. J Anim Ecol 62:1–11
Evison SEF, Fazio G, Chappell P, Foley K, Jensen AB, Hughes WOH (2013) Host-parasite genotypic interactions in the honey bee: the dynamics of diversity. Ecol Evol 3:2214–2222
Eyer PA, Hefetz A (2018) Cytonuclear incongruences hamper species delimitation in the socially polymorphic desert ants of the Cataglyphis albicans group in Israel. J Evol Biol 31:1828–1842
Eyer PA, Freyer J, Aron S (2013a) Genetic polyethism in the polyandrous desert ant Cataglyphis cursor. Behav Ecol 24:144–151
Eyer PA, Leniaud D, Darras H, Aron S (2013b) Hybridogenesis through thelytokous parthenogenesis in two Cataglyphis desert ants. Mol Ecol 22:947–955
Farji-Brener AG, Elizalde L, Fernández-Marín H, Amador-Vargas S (2016) Social life and sanitary risks: evolutionary and current ecological conditions determine waste management in leaf-cutting ants. Proc R Soc B 283:20160625
Frumhoff PC, Schneider S (1987) The social consequences of honey bee polyandry: the effects of kinship on worker interactions within colonies. Anim Behav 35:255–262
Gálvez D, Chapuisat M (2014) Immune priming and pathogen resistance in ant queens. Ecol Evol 4:1761–1767
Ganz HH, Ebert D (2010) Benefits of host genetic diversity for resistance to infection depend on parasite diversity. Ecology 91:1263–1268
Hamilton WD (1964) The genetical evolution of social behaviour I and II. J Theor Biol 7:1–52
Hamilton WD (1982) Pathogens as causes of genetic diversity in their host populations. In: Anderson RM, May RM (eds) Population biology of infectious diseases. Springer-Verlag, New York, pp 269–296
Hamilton WD (1987) Kinship, recognition, disease and intelligence. In Animal societies: theories and facts (eds Ito Y., Brown J.L., et Kikkawa J.). Japan Scientific Societies Press, Tokyo, 291 pp
Hamilton C, Lejeune BT, Rosengaus RB (2011) Trophallaxis and prophylaxis: social immunity in the carpenter ant Camponotus pennsylvanicus. Biol Lett 23:89–92
Hart AG, Ratnieks FLW (2002) Waste management in the leaf-cutting ant Atta colombica. Behav Ecol 13:224–231
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometric J 50:346–363
Hughes WOH, Boomsma JJ (2004) Genetic diversity and disease resistance in leaf cutting ant societies. Evolution 58:1251–1260
Hughes WO, Eilenberg J, Boomsma JJ (2002) Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proc R Soc B 269:1811–1819
Hughes WOH, Oldroyd BP, Beekman M, Ratnieks FL (2008) Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 30:1213–1216
Invernizzi C, Peñagaricano F, Tomasco IH (2009) Intracolonial genetic variability in honeybee larval resistance to the chalkbrood and American foulbrood parasites. Insect Soc 56:233–240
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev 75:21–64
Johansson H, Dhaygude K, Lindström S, Helanterä H, Sundström L, Trontti K (2013) A metatranscriptomic approach to the identification of microbiota associated with the ant Formica exsecta. PLoS ONE 8:e79777
Keller L (1993) Queen number and sociality in insects. Oxford Science Press, Oxford, p 456
Keller L (1995) Social life: the paradox of multiple-queen colonies. Trends Ecol Evol 10:355–360
Konrad M, Vyleta ML, Theis FJ et al (2012) Social transfer of pathogenic fungus promotes active immunisation in ant colonies. PLOS Biol 10:e1001300
Kraus B, Page RE Jr (1998) Parasites, pathogens, and polyandry in social insects. Am Nat 151:383–391
Kuhn A, Bauman D, Darras H et al (2017) Sex-biased dispersal creates spatial genetic structure in a parthenogenetic ant with a dependent-lineage reproductive system. Heredity 119:207–213
Kuhn A, Darras H, Paknia O, Aron S (2020) Repeated evolution of queen parthenogenesis and social hybridogenesis in Cataglyphis desert ants. Mol Ecol 29:549–564
Lee GM, McGee PA, Oldroyd BP (2013a) Variable virulence among isolates of Ascosphaera apis: testing the parasite-pathogen hypothesis for the evolution of polyandry in social insects. Naturwissenschaften 100:229–234
Lee GM, Brown MJF, Oldroyd BP (2013b) Inbred and outbred honey bees (Apis mellifera) have similar innate immune responses. Insect Soc 60:97–102
Leniaud L, Heftez A, Grumiau L, Aron L (2011) Multiple mating and supercoloniality in Cataglyphis desert ants. Biol J Linn Soc 104:866–876
Liersch S, Schmid-Hempel P (1998) Genetic variation within social insect colonies reduces parasite load. Proc R Soc B 265:221–225
Lindström S, Timonen S, Sundström L (2021) The bacterial and fungal community composition in time and space in the nest mounds of the ant Formica exsecta (Hymenoptera: Formicidae). MicrobiologyOpen 10:e1201
Liu L, Zhao XY, Tang QB, Lei CL, Huang QY (2019) The mechanisms of social immunity against fungal infections in eusocial insects. Toxins 11:244
Lowe EC, Simmons LW, Baer B (2011) Worker heterozygosity and immune response in feral and managed honeybees (Apis mellifera). Austral J Zool 59:73–78
Lucas J, Bill B, Stevenson B, Kaspari M (2017) The microbiome of the ant-built home: the microbial communities of a tropical arboreal ant and its nest. Ecosphere 8:e01639
Malagocka J, Eilenberg J, Jensen AB (2019) Social immunity behaviour among ants infected by specialist and generalist fungi. Curr Opin Insect Sci 33:99–104
Masri L, Cremer S (2014) Individual and social immunisation in insects. Trends Immun 35:471–482
Mattila HR, Seeley TD (2007) Genetic diversity in honey bee colonies enhances productivity and fitness. Science 317:632–364
Mattila HR, Rios D, Walker-Sperling VE, Roeselers G, Newton ILG (2012) Characterization of the active microbiotas associated with honey bees reveals healthier and broader communities when colonies are genetically diverse. PLoS ONE 7:e32962
McCallum H, Barlow N, Hone J (2001) How should pathogen transmission be modelled? Trends Evol Ecol 16:295–300
Meurville MP, LeBoeuf A (2021) Trophallaxis: the functions and evolution of social fluid exchange in ant colonies (Hymenoptera: Formicidae). Myrmecol News 31:1–30
Meyling NV, Eilenberg J (2007) Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol Cont 43:145–155
Oldroyd BP, Clifton MJ, Parker K, Wongsiri S, Rinderer TE, Crozier RH (1998) Evolution of mating behavior in the genus Apis and an estimate of mating frequency in A. cerana (Hymenoptera: Apidae). Ann Entomol Soc Am 91:700–709
Palmer KA, Oldroyd BP (2000) Evolution of multiple mating in the genus Apis. Apidologie 31:235–248
Palmer KA, Oldroyd BP (2003) Evidence for intra-colonial genetic variance in resistance to American foulbrood of honey bees (Apis mellifera): further support for the parasite ⁄ pathogen hypothesis for the evolution of polyandry. Naturwissenschaften 90:265–268
Pereira H, Jossart M, Detrain C (2020) Waste management by ants: the enhancing role of larvae. Anim Behav 168:187–198
Pérez-Lachaud G, Valenzuela JE, Lachaud JP (2011) Is increased resistance to parasitism at the origin of polygyny in a Mexican population of the ant Ectatomma tuberculatum (Hymenoptera: Formicidae)? Florida Entomol 94:677–684
Qiu H, Lu L, Shi Q et al (2014) Fungus exposed Solenopsis invicta ants benefit from grooming. J Insect Behav 27:678–691
R Core Team (2014) R version 3.1.1: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rauch G, Kalbe M, Reusch TBH (2007) Partitioning average competition and extreme genotype effects in genetically diverse infections. Oikos 117:399–405
Reber A, Castella G, Christe P, Chapuisat M (2008) Experimentally increased group diversity improves disease resistance in an ant species. Ecol Lett 11:682–689
Rheindt FE, Strehl CP, Gadau JA (2005) Genetic component in the determination of worker polymorphism in the Florida harvester ant Pogonomyrmex badius. Insect Soc 52:163–168
Rosengaus RB, Traniello JFA, Chen T, Brown JJ, Karp RD (1999) Immunity in a social insect. Naturwissenschaften 86:588–591
Rosengaus RB, Malak T, MacKintosh C (2013) Immune-priming in ant larvae: social immunity does not undermine individual immunity. Biol Lett 9:20130563
Rueppell O, Johnson N, Rychtar J (2008) Variance-based selection may explain general mating patterns in social insects. Biol Lett 4:270–273
Sadd BM, Schmid-Hempel P (2006) Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr Biol 16:1206–1210
Saga T, Okuno M, Loope KJ, Tsuchida K et al (2020) Polyandry and paternity affect disease resistance in eusocial wasps. Behav Ecol 31:1172–1179
Schmid-Hempel P (1998) Parasites in social insects. Monographs in Behavior and Ecology. Princeton University Press, Princeton, 392 pp
Schmid-Hempel P, Crozier RH (1999) Polyandry versus polygyny versus parasites. Philos Trans R Soc b: Biol Sci 354:507–515
Schmidt AM, Linksvayer TA, Boomsma JJ, Pedersen JS (2011) No benefit in diversity? The effect of genetic variation on survival and disease resistance in a polygynous social insect. Ecol Entomol 36:751–759
Seeley TD, Tarpy DR (2007) Queen promiscuity lowers disease within honeybee colonies. Proc R Soc b: Biol Sci 274:67–72
Sherman PW, Seeley TD, Reeve HK (1988) Parasites, pathogens, and polyandry in social Hymenoptera. Am Nat 131:602–610
Simone-Finstrom M, Walz M, Tarpy DR (2016) Genetic diversity confers colony-level benefits due to individual immunity. Biol Lett 12:20151007
Slayter RA, Mautz BS, Backwell PRY, Jennions MD (2012) Estimating genetic benefits of polyandry from experimental studies: a meta-analysis. Biol Rev 87:1–33
Strassmann J (2001) The rarity of multiple mating by females in the social Hymenoptera. Insect Soc 48:1–13
Tarpy DR (2003) Genetic diversity within honeybee colonies prevents severe infections and promotes colony growth. Proc R Soc b: Biol Sci 270:99–103
Tarpy DR, Seeley TD (2006) Lower disease infections in honeybee (Apis mellifera) colonies headed by polyandrous vs monandrous queens. Naturwissenschaften 93:195–199
Therneau T (2011) Survival: survival analysis, including penalised likelihood. http://cran.r-project.org/web/packages/survival
Tranter C, Graystock P, Shaw C et al (2014) Sanitizing the fortress: protection of ant brood and nest material by worker antibiotics. Behav Ecol Sociobiol 68:499–507
Ulgevig LV, Cremer S (2007) Social prophylaxis: group interaction promotes collective immunity in ant colonies. Curr Biol 17:1967–1971
Ulgevig LV, Kronauer DJC, Schrempf A, Heinze J, Cremer S (2010) Rapid anti-pathogen response in ant societies relies on high genetic diversity. Proc R Soc b: Biol Sci 277:2821–2828
van Baalen M, Beekman M (2006) The costs and benefits of genetic heterogeneity in resistance against parasites in social insects. Am Nat 167:568–577
Van Valen L (1973) A New Evolutionary Law. Evol Theor 1:1–30
Walsh PS, Metzger DA, Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10:506–513
Wang JL (2002) An estimator for pairwise relatedness using molecular markers. Genetics 160:1203–1215
Wang JL (2004) Sibship reconstruction from genetic data with typing errors. Genetics 166:1963–1979
Westhus C, Ugelvig LV, Tourdot E et al (2014) Increased grooming after repeated brood care provides sanitary benefits in a clonal ant. Behav Ecol Sociobiol 68:1701–1710
Wiernasz DC, Perroni CL, Cole BJ (2004) Polyandry and fitness in the western harvester ant, Pogonomyrmex occidentalis. Mol Ecol 131:1601–1606
Wiernasz DC, Hines J, Parker DG, Cole BJ (2008) Mating for variety increases foraging activity in the harvester ant, Pogonomyrmex occidentalis. Mol Ecol 17:1137–1144
Wilson EO (1992) The effects of complex social life on evolution and biodiversity. Oikos 63:13–18
Wilson-Rich N, Spivak M, Fefferman NH, Starks PT (2009) Genetic, individual, and group facilitation of disease resistance in insect societies. Ann Rev Entomol 54:405–423
Wilson-Rich N, Tarpy DR, Starks PT (2012) Within- and across-colony effects of hyperpolyandry on immune function and body condition in honey bees (Apis mellifera). J Insect Physiol 58:402–407
Yasui Y (1998) The ‘genetic benefits’ of female multiple mating reconsidered. Trends Ecol Evol 13:246–250
Acknowledgements
Thanks to M. Avet for her help with experiments. Thanks to D. Gàlvez and M. Chapuisat for providing fungus samples, their helpful experimental expertise, and their comments on previous versions of the manuscript. We are grateful to B. Gassner for English improvement of the manuscript, as well as to the Morocco’s High Commission for Water, Forests and Combating Desertification (HCEFLCD—Decision 277/2012) for granting us collection permits. This work was supported by a PhD fellowship from the FRIA (Fonds pour l’Encouragement de la Recherche Scientifique dans l’Industrie et l’Agriculture) (PAE), as well as grants from the Belgian FRS-FNRS (Fonds National pour la Recherche Scientifique; grants # T.0140.18 and J.0063.14) and the Université Libre de Bruxelles (Actions Blanches) (SA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Communicated by B. Baer
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eyer, P.A., Guery, P.A. & Aron, S. Genetic diversity, paternal origin and pathogen resistance in Cataglyphis desert ants. Behav Ecol Sociobiol 77, 81 (2023). https://doi.org/10.1007/s00265-023-03358-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03358-y