Abstract
Marek’s disease virus (MDV) is a highly pathogenic and oncogenic alpha herpesvirus that causes Marek’s disease (MD), which is one of the most important immunosuppressive and rapid-onset neoplastic diseases in poultry. The onset of MD lymphomas and other clinical diseases can be efficiently prevented by vaccination; these vaccines are heralded as the first demonstration of a successful vaccination strategy against a cancer. However, the persistent evolution of epidemic MDV strains towards greater virulence has recently resulted in frequent outbreaks of MD in vaccinated chicken flocks worldwide. Herein, we provide an overall review focusing on the discovery and identification of the strategies by which MDV evades host immunity and attacks the immune system. We have also highlighted the decrease in the immune efficacy of current MD vaccines. The prospects, strategies and new techniques for the development of efficient MD vaccines, together with the possibilities of antiviral therapy in MD, are also discussed.
Similar content being viewed by others
Introduction
Marek’s disease (MD) is a highly contagious and rapidly progressive lymphoproliferative avian disease, that is caused by pathogenic Marek’s disease virus (MDV) and is characterized by severe immunosuppression, neurological disorders and malignant T-cell lymphomas1. In addition to the direct effects of the disease on mortality, MDV infection also causes secondary infections, decreased productivity, and increased morbidity. The causative agent of MD is gallid alphaherpesvirus 2 (GaAHV-2), which is also known as MDV type 1 (MDV-1), a prototypic member of the genus Mardivirus of the Alphaherpesvirinae subfamily that includes other associated avian herpesviruses, such as gallid alphaherpesvirus 3 (GaAHV-3)/MDV type 2 (MDV-2) and meleagrid alphaherpesvirus 1 (MeAHV-1)/turkey herpesvirus (HVT)2. Although they are antigenically related to MDV-1, MDV-2 and HVT are both non-pathogenic viruses in chickens. Based on their virulence and pathogenicity to birds, MDV-1 isolates can be further divided into distinct pathotypes, including mild (m), virulent (v), very virulent (vv) and very virulent plus (vv + ) MDVs3. As the first tumour disease that can be prevented by vaccination, MD has been well controlled in the 21st century through large-scale vaccination programs4. However, the evolution of epidemic MDVs towards higher virulence, especially the newly emerged vv+MDV and hypervirulent MDV (HV-MDV) variants driven by long-term MD vaccination and imperfect immunization, has recently caused frequent outbreaks of MD in vaccinated chicken flocks worldwide5,6,7,8,9.
Since the 1970s, three widely used MD vaccines, including the FC-126 strain of HVT, the SB-1 strain of MDV-2, and the CVI988/Rispens (CVI988) strain of MDV-1, have been developed for the efficient control of disease10. Over the past several decades, CVI988 has been regarded as the most effective vaccine against MDV, but the underlying mechanisms that improve protective immunity following vaccination are not fully understood. Along with the persistent evolution of viruses, specific changes in MDV genomes have been documented, possibly contributing to the increase in virulence and allowing the virus to overcome MD vaccine protection9,11,12,13,14. Unfortunately, to date, there are few alternative vaccines, aside from CVI988, that can be used to combat the emerging HV-MDV variants5,15.
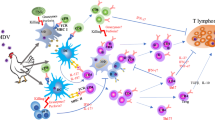
MDV invades host immune cells such as macrophages and lymphocytes16,17, leading to severe immunosuppression and resulting in weaker protection mediated by MD vaccination. As summarized in Fig. 1, MDV can evade host surveillance and immune responses via several mechanisms to provide a favourable environment for self-replication and proliferation. For example, MDV reduces the expression of viral immunogenic antigens through semi-productive cytolytic replication18 and downregulates the expression of major histocompatibility complex class I (MHC-I) on infected lymphocytes to achieve immune evasion19. The regulation of both viral and host cellular genes involved in immunity during MDV infection occurs at both the transcriptional and posttranslational levels to limit host immune responses to the virus20,21. MDV also encodes proteins that interfere with and modulate the host immune system, thus allowing viruses to establish lifelong persistent infection22. In addition to viral proteins, noncoding RNA genes (ncRNAs) are also involved in the MDV lifecycle, oncogenesis, and even immunoregulation23. In particular, some MDV-1-encoded microRNAs (miRNAs) recognize both viral and/or cellular genes to evade host innate and acquired immune mechanisms through epigenetic control and/or posttranslational regulation of mRNA targets. As one of the most virulent oncogenic herpesviruses, MDV has mastered the exploitation and modulation of host immune function. However, MDV-induced immunosuppression is very complex, poorly understood, and in many cases unrevealed. An overall review of the mechanisms of immune evasion and strategies of MDV evasion of the host immune system, together with a glance at the immune efficacy of widely used MD vaccines and the new generation of gene-editing technology, will be meaningful for the future development of novel MD vaccines and the efficient control of this disease.
The key strategies and potential approaches, such as modulation of histocompatibility complexes, regulation of virus replication, viral telomeric integration, inhibition of interferon expression, manipulation of macrophages, suppression of NK-cell activation, disruption of mitochondrial dynamics, modulation of humoral immunity, and evasion of adaptive immunity, are drawn together to demonstrate the intricate interactions between viral components, host cellular proteins, and/or non-coding RNAs to counteract the host immune response. (Created with Microsoft PowerPoint).
Host immune responses to MDV infection
MDV infection induces a comprehensive host immune response that includes the innate and acquired immune responses; this response includes cytokine release, immune cell recruitment, and changes in the expression patterns of host immune-related genes. The degree of immune response induced by the virus is quite different in hosts with different levels of MD resistance/susceptibility. Transcriptome analysis of bone marrow-derived macrophages and dendritic cells from susceptible or resistant chickens infected with MDV revealed a higher viral infection rate and stronger activation of the immune system in susceptible birds24,25, indicating that the development of MD resistance likely occurs during the early stage of infection through the innate immune response. Increased expression of key immune genes was detected in the innate immune cells from the resistant chickens compared to those from the susceptible chickens, thus showing inherent immune supremacy. However, after MDV challenge, only a limited number of immune-related genes, such as iNOS, which is thought to inhibit MDV replication by releasing NO, were highly expressed in the innate immune cells of the resistant chickens. Conversely, the expression of a number of genes involved in immune pathways, such as TLR and JAK-STAT signalling, were downregulated in the resistant chickens. On the one hand, this may be attributed to virus evasion of immune detection, consistent with the idea that MDV induces a latent phase in resistant birds. On the other hand, this approach could represent a defensive strategy employed by resistant hosts to disrupt the actin-mediated propagation of MDV between cells through the inactivation of immune pathways, thereby limiting MDV infection.
MDV also regulates the expression patterns of host immune-related genes at different stages of infection to facilitate pathological progression. A previous study showed that during the latent stage, vv+MDV induced measurable suppression of gene expression associated with host defence, but this suppression was followed by apparent activation of the defence response during viral reactivation in susceptible chickens21. Functional enrichment analysis of MDV-induced differentially expressed genes (DEGs) revealed that at 10 days post infection (dpi), a large number of genes that had downregulated expression in the susceptible line 72 were significantly enriched in several terms associated with fatty acid metabolism, which may suppress virus replication and control cancer cell proliferation to play a role in establishing latency or repressing MDV infection. Only a few genes that had upregulated expression were enriched in two GO terms, immune response and immune effector process. However, at 21 dpi, 85% of the DEGs had upregulated expression and were significantly enriched in immune response-related terms, implying that a strong defence response was activated and ready to control tumorigenesis and disease progression during the reactivation stage. Interestingly, the expression of the immune factors IFN-α and IFN-β in the thymus and bursa of Fabricius was downregulated during the cytolytic infection and reactivation stages of MDV infection, but not during the latent stage26.
Notably, the differential expression of immune-related genes may also be related to the virulence and pathogenicity of distinct MDV strains. Using RNA sequencing (RNA-seq), transcriptomes of bursa and spleen lymphocytes from CVI988- or vvRB1B-challenged chickens preliminarily revealed differential immune responses initiated by vaccine or virulent MDV strains during early cytolytic infection27. The identification of highly significant genes associated with host immune competence showed that lymphocytes from CVI988-vaccinated chickens could elicit a stronger early immune response than lymphocytes from vvRB1B-infected chickens. Notably, the expression of IL-1β, IL-6, IL8L1, CCL4 and CCL5 was significantly upregulated in splenic lymphocytes from CVI988-infected birds compared to those from vvRB1B-infected birds; this experiment provided valuable data on the transcriptional landscape to understand vaccine-mediated immune protection mechanism.
Immune evasion during different phases of virus infection
Modulation of major histocompatibility complexes
Generally, herpesviruses can modulate the expression of both MHC-I and MHC-II molecules in infected immune cells, such as macrophages and B cells, to evade host cell-mediated immunity. The MHC gene family encodes immunity-related cell surface molecules that are primarily involved in antigen presentation and the regulation of T lymphocytes and impact the specificity of the immune response to virus- and MD-transformed cells. Chicken MHC haplotypes differ in their ability to present various kinds of peptides due to MHC-peptide interactions, and these differences may be responsible for MHC-related resistance to MD28. All MDV serotypes are reported to reduce the surface expression of MHC-I glycoproteins during viral lytic replication, which is believed to facilitate immune evasion by the virus19. Further studies have demonstrated that two MDV genes, MDV012 and pUL.49.5, which are listed in Table 1, play a role in the downregulation of MHC-I molecule expression by interfering with the function of transporters associated with antigen presentation (TAPs)29,30. Compared to those of the MHC-I system, few studies have investigated chicken MHC-II genes and molecules. The vast majority of MDV peptide epitopes presented by chicken MHC-II molecules arise from only four viral proteins, namely, glycoproteins gH, gI, and gE and the UL43 tegument protein31. MDV infection-related up- or downregulation of MHC-II expression depends on both the genetic background of the chicken host and the strain of virus32,33. The downregulation of MHC-II expression caused by MDV infection could be easily associated with viral immune escape, but the role of the upregulation of MHC-II expression in the immunopathological response needs to be further investigated.
Self-inhibition of viral activity during latency
The presence of latent MDV particles in host cells usually occurs at approximately 7 dpi, primarily in CD4+ T lymphocytes of virus-challenged birds. During the latent phase, expression of the viral genome is limited to only some specific genes that are required to maintain latency, whereas no progeny virions are produced, and the latent virus effectively evades host immune system surveillance18. As listed in Table 1, the currently known MDV latency-related genes located in the long and short repeat regions of the viral genome include the major oncogene meq, the viral telomerase RNA (vTR), latency-associated transcripts (LATs) and a number of viral miRNAs.
The meq gene encodes a basic leucine zipper transcription factor with favoured dimerization with the oncoprotein c-Jun (Meq/c-Jun) and itself (Meq/Meq). The binding of Meq/c-Jun to AP-1-containing promoters facilitates the survival of latently infected cells through activation of the expression of the antiapoptotic factor Bcl-234. Binding of the homodimer Meq/Meq to the MERE II site of the MDV replication origin suppresses transcription from flanking bidirectional promoters (pp38/24 and pp14)35, thus potentially impeding the expression of early lytic genes, such as pp38. This transcriptional regulation may hinder viral replication and productive infection; thus, this regulation warrants further investigation. Meq may also help maintain latent MDV infection in combination with other transcription factors through the modification of host and viral gene expression20,36. MDV is the only virus known to encode a telomerase RNA subunit (vTR), and MDV vTR shares 88% sequence identity with chicken telomerase RNA (chTR). vTR mainly serves as a template for the addition of telomeric repeats and interacts with chicken telomerase reverse transcriptase (TERT) to participate in MDV integration37. Moreover, vTR has also been speculated to have antiapoptotic effects on latently infected cells37,38. LATs are a family of long ncRNAs (~10 kb) that are expressed mainly during virus latency and lymphomagenesis, suggesting that they play a role in the maintenance of latency and/or cell transformation. Small ncRNAs, such as MDV-1 miRNAs, have also been demonstrated to target both viral and cellular mRNAs to promote long-term persistent infection. For example, miR-M7-5p has been verified to target the MDV-1 immediate-early genes ICP4 and ICP27, thus contributing to the establishment and maintenance of latency39. miR-M5-3p, another MDV-1 miRNA, is also reported to downregulate the expression of viral infected cell protein 22 (ICP22), which is required for the lytic replication of viruses40. miR-M4-5p, a viral orthologue of cellular oncogenic miR-155 that plays a crucial role in MDV tumorigenesis, can directly modulate the expression of viral targets such as UL28 and UL3241,42. Thus, maintaining viral latency has also been suggested as a potential mechanism for miR-M4-5p43.
In addition to viral genes, some host proteins may be hijacked to help establish MDV latency. Ikaros (IKFZ1) has recently been validated as a cancer driver gene for MD lymphomas44, and it is speculated to be involved in latent MDV infection because of its important role in regulation of the herpesvirus latent-lytic switch45.
Telomeric integration and MDV immune escape
In latently infected cells and tumour cells, MDV specifically integrates its genome in the telomeric region of host chromosomes. MDVs may use integration not only to avoid host immunosurveillance and maintain latency but also to maintain persistence in host chromosomes, viral genome replication, and host cell proliferation46. Furthermore, telomeres repress the expression of adjacent genes, which is beneficial for silencing the virus genome during the establishment of latency. As listed in Table 1, there is a growing amount of data on the viral components involved in the integration process, but the exact mechanism and factors required for MDV integration are not fully understood47.
MDV harbours two viral telomeric repeat (TMR) arrays, multiple telomeric repeats (mTMR) and short telomeric repeats (sTMR), which are identical to the host telomere sequence of TTAGGG. The viral TMR is hypothesized to serve as a mini-chromosome cap or contribute to the ability of the viral genome to integrate into the host genome via recombination or a telomerase-based pathway. Both mTMR and sTMR have been shown to facilitate integration of the MDV genome into host telomeres during latency48,49. The expression of vTR is regulated by DNA methylation patterns in functional c-Myc response elements (REs) of the vTR promoter, thereby controlling MDV integration through vTR-mediated telomerase activity50,51. The absence of a cell population exhibiting a telomere-integrated phenotype following infection with the Δmeq MDV strain suggests that the oncogene meq plays a role in facilitating telomeric integration of MDV in activated lymphocytes52. Considering the transcriptional regulation of vTR by c-Myc through the functional c-Myc RE within the vTR promoter, we speculate that Meq indirectly regulates vTR through c-Myc expression, which is induced by Meq/c-Jun heterodimers, thereby enhancing the function of viral telomere integration34,51. miR-M4-5p may also participate in MDV integration as it has been demonstrated to target the TGF-β signalling pathway by downregulating LTBP1 expression to activate the expression of c-Myc53. In addition, the VP22-induced DNA lesions observed at the early stage of virus infection in vivo may facilitate MDV integration directly or indirectly by triggering the DNA damage response (DDR) and DNA repair pathways, especially homologous recombination54,55. Recently, based on a quantitative integration assay using chicken T-cell lines as targets for MDV latency and transformation, a novel cell culture system for in vitro integration has been developed to provide a tool for revealing the mechanism of MDV integration into host telomeres in the future56.
Immune damage and immunosuppression of the host immune system
MDV induces serious immunosuppression (IS) through a variety of mechanisms during pathogenesis, and IS is often divided into two phases: an early IS phase associated with early cytolytic infection of lymphoid organs and a late IS phase associated with the reactivation of MDV and the development of tumours57. The stage of MDV-induced IS involves the viral component-mediated regulation of host innate and adaptive immune responses (Table 2).
Viral strategies to combat innate immunity
Upon viral infection, host cells recognize pathogenic microorganisms through pattern recognition receptors (PRRs) to activate the first defence system, namely, the innate immune system, to eliminate pathogenic microorganisms or activate the adaptive immune response. As a smart pathogen, MDV has also evolved various strategies to evade the innate immune system.
Interferons (IFNs)
IFNs are widely expressed cytokines that possess strong antiviral and immunomodulatory properties and are mainly classified into three types: type I (IFN-α and IFN-β), type II (IFN-γ) and type III (IFN-λ). The role of IFNs in MDV replication has been confirmed through both in vitro and in vivo models, in which IFNs not only reduce the plaque formation and expression of pp38 and gB in virus-infected cells but also significantly delay the onset and progression of disease58. The protective role of IFNs is also indicated by the differential expression patterns of the IRF3 and IFN-β genes in resistant and susceptible chicken lines59. However, the expression of IFNs is significantly decreased in the thymus and bursa of Fabricius in MDV-challenged birds at the lytic infection stage, implying that MDV inhibits IFN-I expression in the host and results in immunosuppression26. One of the important strategies by which MDV evades the IFN-mediated innate immune response is to hijack host proteins involved in the IFN signal transduction pathway. cGMP-AMP synthase (cGAS), which is an important cytosolic DNA sensor, detects cytosolic viral DNA via stimulator of interferon genes (STING) to initiate the innate antiviral response, indicating that the cGAS-STING pathway plays an important role in the host response to MDV infection20. As demonstrated in Fig. 2, five MDV proteins, namely, Meq, RLORF4, US3, UL46 and VP23, reduce IFN-β induction by regulating the cGAS-STING pathway. Meq inhibits STING signalling by impairing the assembly of the STING-TBK1-IRF7 complex, thereby preventing IRF7 activation and the induction of IFN-β. Deletion of the transactivation domain of Meq may impact its immunosuppressive effects by disrupting the interaction between STING and the C-terminal domain of Meq, consequently leading to a reduction in viral pathogenicity20. RLORF4 was found to suppress nuclear translocation of the endogenous NF-κB subunits p65 and p50, which ultimately blocks IFN-β promoter activation induced by cGAS and STING60. The other two viral proteins, US3 and VP23, were both shown to suppress interferon stimulatory DNA (ISD)-triggered IFN-β production by blocking the nuclear translocation of IRF7 through different strategies61,62. As a multifunctional serine/threonine protein kinase, US3 is also involved in the regulation of various cellular pathways by regulating the transcriptional function of the CREB/Meq heterodimer in a phosphorylation-dependent manner, which influences cellular and viral gene expression63. In addition to functioning as cytosolic DNA sensors, Toll-like receptors (TLRs) also function in the induction of proinflammatory cytokine and IFN-β production in the host immune response to MDV infection64,65. Strikingly, MDV infection also triggers Dead-box Helicase 5 (DDX5) to thwart the activation of IFN-β by blocking Toll-like receptor 3 (TLR3) signalling cascades; this result provides new insight into the mechanism by which viruses hijack host proteins to achieve immunosuppression66.
MDVs exploit viral proteins and noncoding RNAs to evade host innate immunity by inhibiting the cGAS-STING signaling pathway, the MDA5-mediated signaling pathway and the Toll-like receptor signaling pathway. cGAS, cyclic GMP-AMP synthase; cGAMP, cyclic GMP-AMP; STING, stimulator of interferon genes; MDA5, melanoma-differentiation-associated gene 5; MAVS, mitochondrial antiviral signaling protein; TLR3, Toll-like receptor 3; TRIF, TIR-domain-containing adapter inducing interferon-β; TRAF, tumor necrosis factor receptor associated factor; TBK1, TANK-binding kinase 1; IKKε, nuclear factor kappa-B kinase epsilon; IRF, interferon regulatory transcription factor; NF-κB, nuclear factor-kappa B; NEMO, nuclear factor (NF)-kappa B essential modulator; IKKα, NF-kappa B kinase subunit alpha; IKKβ, nuclear factor kappa-B kinase beta; IκBα/β, nuclear factor kappa-B-α/β; ADAR1, adenosine deaminase acting on RNA1; DDX5, Dead-box Helicase 5. (Created with Microsoft PowerPoint).
MDV also encodes viral miRNAs or regulates host miRNAs to target host proteins and inhibit IFN-regulated host antiviral responses. miR-M4-5p has a wide range of effects on the cellular environment and the global gene expression of lymphocytes by targeting transcription factors such as PU.1, which plays an important role in immune regulation. miR-M4-5p enhances the expression of adenosine deaminase, which acts on RNA1 (ADAR1), thereby inducing hyper-editing of edited repeat long (ERL) lncRNA67. Hyper-edited ERL lncRNAs are hypothesized to disrupt the innate immune system by impeding the activity of MDA5, as the binding of inosine-containing RNAs by MDA-5 is known to inhibit activity and to block IFN expression68,69. Targeting and regulating the TLR3-mediated signalling pathway via miR-M4-5p is another way for MDV to inhibit the host innate immune response70. In addition, both miR-M2-3p and miR-M9-5p were predicted to target IL-18, which is a proinflammatory cytokine that stimulates IFN-γ production; this targeting may allow the virus to evade host defences71. Some cellular miRNAs, such as gga-miR-155, have also been shown to help viruses evade the host innate immune system72. Interestingly, MDV is known to replace and downregulate miR-155 expression in host cells through its own orthologue, miR-M4-5p, thereby exploiting the regulatory pathways of miR-155 by effectively modulating the targets associated with this miRNA71,73. Notably, viral miRNA-mediated inhibition of host miRNA processing represents a ubiquitous cellular mechanism by which herpesviruses disrupt intrinsic host immunity 74; this mechanism will be a very intriguing focus for future research on MDV pathogenesis.
Innate immune cells
Innate immune cells are composed mainly of phagocytes and nonphagocytic cells. Among them, macrophages and dendritic cells, as antigen-presenting cells (APCs) involved in the initiation of the immune response, play important roles in the development of immunity against MDV. The role of dendritic cells in MD immunology is quite limited, but they are thought to be involved in the initiation of host innate and adaptive immunity25. The role of macrophages in anti-MDV infection can be determined based on vaccination-activated macrophages and the increase in macrophage populations within the duodenum in MD-resistant and MD-susceptible chickens75. In addition to presenting MDV antigens in association with MHC class I and II molecules to initiate adaptive immunity, macrophages are also directly involved in the inhibition of MDV replication and disease development. Unfortunately, macrophages become infected either directly or after an initial round of MDV replication, which leads to macrophage death and heavy infiltration around blood vessels. The virus can also replicate in macrophages, making macrophages excellent candidates for transporting MDV to primary and secondary lymphoid organs. Moreover, macrophage migration inhibitory factor (MIF), a soluble mediator secreted by activated T cells that inhibits the migration of macrophages, was reported to be involved in MD pathogenesis76. On the one hand, decreased expression of MIF during early and latent infection enhances macrophage migration, which might be a potential mechanism by which MDV increases virus transport and spread. On the other hand, upregulation of MIF expression after latent infection might be employed by MDV to induce lymphoma occurrence. First, MIF sustains macrophage survival and function by suppressing p53-dependent apoptosis, which is important for MDV-infected macrophages to spread the virus77. Second, MIF exerts significant protumour effects by regulating antitumour T lymphocyte responses78. Compared to activated and fully functional macrophages, tumour-associated macrophages (TAMs) produce tumour growth-promoting factors and induce immunosuppression by releasing immunomodulatory factors79. Immunoregulatory macrophages isolated from MDV-infected chickens exhibit abilities similar to those of TAMs and are correlated with transient immunosuppression during the primary cytolytic phase of infection.
Natural killer (NK) cells are key players in the innate immune response and can respond to stimuli and produce antiviral cytokines such as IFN-γ. These cells recognize virus-infected cells via the ligation of cell death receptors and the release of granules. The various viral strategies used to modulate the recognition of herpesviruses indicate the importance of NK cells in the control of such viruses80,81. The NK cells in MDV-infected chickens are more active than those isolated from uninfected birds, and in resistant chickens, this activity lasts longer than that in susceptible chickens. However, the mechanisms of NK cell evasion by MDV have largely remained elusive. MDV reduced the expression of the MHC molecule BF2 and prevented the downregulation of BF1 expression; BF1 specifically interacts with NK cells, thereby inhibiting NK cell activation82. Recent data have also provided evidence that NK cells can be efficiently infected by MDV and that cell activation is dependent on the expression of the major oncogene meq, thus providing additional evidence for NK cell-related immune regulation mediated by MDV83.
Mitochondria
Mitochondria are crucial cellular organelles in eukaryotes and participate in many cell processes, including the immune response, growth development, and tumorigenesis. Viral infection can activate or inhibit mitochondrial function, alter mitochondrial contents, and influence gene expression. Mitochondrial DNA (mtDNA) is normally present at thousands of copies per cell and is packaged into several hundred higher-order structures termed nucleoids84, and mtDNA stress elicited by herpesviruses engages the DNA sensor cGAS and promotes STING-IRF3-dependent signalling to increase ISG expression, potentiate IFN-I responses, and confer viral resistance85. Moreover, alphaherpesviruses have evolved mechanisms to disrupt mitochondrial dynamics, cause mitochondrial dysfunction and enhance viral infection and pathogenesis86. HVT-encoded vNr-13, the first known alphaherpesvirus-encoded Bcl-2 homologue of Nr-13, localizes to the mitochondria and endoplasmic reticulum (ER) and disrupts mitochondrial network morphology in virus-infected cells to modulate the host immune response87. MDV infection results in an imbalance in mitochondrial content and gene expression, especially significantly reduced levels of mtDNA in the spleens of MD-susceptible 72 birds, suggesting that mitochondrial dysfunction is indispensable for virally induced cell transformation and subsequent disease development in chickens88. MDV-induced mtDNA stress is necessary for effective ISG expression and antiviral priming, but mtDNA damage can also lead to inflammation and apoptosis and ultimately trigger oncogenesis in chickens.
Viral strategies to combat adaptive immunity
The key components of adaptive immunity are B and T lymphocytes, which are involved in tightly regulated interactions with antigen-presenting cells and facilitate pathogen-specific immunologic effector pathways, generate immunologic memory, and regulate host immune homeostasis. B cells are involved in the humoral immune response, whereas T cells are involved in the cell-mediated immune response. The early pathogenesis of MDV is characterized by a burst of productive/restrictive infection in B cells, followed by a latent infection in activated CD4+ T cells that can persist for three weeks prior to reactivation and transformation38. Destruction of B and T cells during lytic infection ultimately leads to severe atrophy of immune organs and consequential immunosuppression in hosts17.
Humoral immunity
Due to the strict cell-associated nature of MDV, B-cell-mediated humoral immunity may be limited to provide protection against the virus. However, various studies on host responses to MDV infection in the bursa have shown that the complete removal of B cells by surgical bursectomy strongly influences MDV-induced pathogenesis and reduces vaccine-mediated immune protection89. The role of humoral immunity in controlling MDV infection has also been confirmed by the presence of maternal antibodies that delay the development of clinical signs and tumours38. However, recent research has shown that B cells do not play an essential role in vaccine-mediated immunity in response to oncogenic MDV strains90. Although the antiviral capacity of B cells during MDV infection needs to be further investigated, it has been well documented that B cells are hijacked by MDV to promote the development of disease. B cells are the primary target cells by which MDV is transported to secondary lymphoid tissues after phagocytosis by macrophages, resulting in early cytolytic infection in the lungs. The lytic activity of MDV-1 pp38 in B cells results in cell apoptosis, the atrophy of lymphoid organs, and a significant decrease in antibody production and antiviral defence, which ultimately causes host immunosuppression17. The route by which MDV particles spread from infected B cells to activated T cells and different organs leading to systemic infection should not be overlooked, although the presence of B cells acting as the initial targets of MDV infection has recently been verified to not be an essential step in the activation and consequent infection of a larger number of CD4+ T cells90,91. Interestingly, in addition to inducing the apoptosis of uninfected B cells, leading to immunosuppression, MDV prolongs the survival of infected B cells by inducing a senescence-like phenotype, which may promote the persistence of viruses and thus allow more time to recruit and infect T cells92. Strikingly, MHC-II molecules, which are embedded in the membranes of APCs such as macrophages, dendritic cells, and B cells, play a crucial role in activating B-cell proliferation and differentiation93. The modulation of MHC-II by MDV mentioned above might contribute to the limitations in humoral immunity during MDV infection and/or vaccine-induced protection. Taken together, these findings indicate that MDV hijacks B cells in different ways, not only by using them as carriers for virus transmission and diffusion but also by limiting the humoral immune response of the host.
Cell-mediated immunity
MDV is a strict cell-associated herpesvirus, and its transmission in vivo depends on cell-to-cell contact; thus, T-cell-mediated immunity is thought to play a more crucial role than the antibody-mediated response. Both antiviral and antitumour T-cell immunity are induced by MDV or MD lymphoblastoid cells to suppress viral replication and prevent the development of tumours, respectively94. While T-cell-mediated immune responses play a pivotal role in combating viral infections, MDV has evolved and developed sophisticated mechanisms for the suppression and evasion of cellular and soluble effectors of the adaptive immune system.
T-helper cells (CD4+) not only act as a bridge between specific APCs and B cells and CD8+ T cells but also help these cells through interactions between CD40 and CD40L, thereby promoting the activation of cytotoxic CD8+ T cells and facilitating the survival, proliferation, and immunoglobulin class switching of B cells. MDV transforms CD4+ T cells approximately three weeks post infection and subsequently leads to atrophy of the thymus, the apoptosis of infected T cells and the development of MD lymphomas38. In addition, the expression of PD-1 was increased on CD4+ T cells of virus-infected birds at 21 dpi95, which prevented T cells from killing tumour cells or infected target cells in the setting of persistent infection and cancer96. Compared to those in CD4+ T cells in the control group, MDV altered the ubiquitylome associated with signal transduction and the immune system, cancer, and infectious disease pathways in CD4+ T lymphoma cells; this result may facilitate future studies on the mechanisms underlying MDV-induced immunosuppression and tumorigenesis in T cells in relation to the regulation of ubiquitination97.
Regulatory T cells (Tregs) are a subset of CD4+ T cells that exert immunoregulatory effects via the secretion of immunosuppressive soluble factors such as IL-10 and TGF-β, and cell contact-mediated regulation through costimulatory molecules such as cytotoxic T lymphocyte antigen 4 (CTLA-4). The immunosuppressive role of Treg cells has been indicated by the increased expression of IL-10 and CTLA-4 in CD4+ T cells during MDV infection, and this effect was more pronounced in MDV-susceptible chickens95,98. The MDV-encoded viral IL-8 (vIL-8) exhibits specific binding affinity for Treg cells (CD4+CD25+ T cells), potentially facilitating their recruitment as a target for MDV infection and subsequent transformation99. In addition, a novel subset of Treg cells expressing TGF-β on the surface was identified in different lymphoid tissues. The frequency of this population was greater in the spleens of MDV-susceptible chicken lines than in those of the resistant line, suggesting that this population plays an important role in MDV pathogenesis and immunosuppression100. Recently, the presence of CD4+CD25+ and CD4+ TGF-β+ Treg cells in the feathers of infected chickens has also demonstrated the ability of MDV to induce an immunomodulatory microenvironment in chicken feathers to achieve immune escape101.
Given that CD4+ cells are primarily hijacked by MDV to promote disease development and tumour formation, other populations of T cells, such as CD8+ and γδ T cells, may play crucial roles in the immune response to MDV infection. The important role of CD8+ T cells has been demonstrated by depleting CD8+ cells using monoclonal antibodies, which increased the likelihood of tumour occurrence and decreased vaccine immune protection against MD102. Subsequent studies have revealed a direct relationship between the magnitude of the T-cell response to MDV-1 antigens (pp38 and Meq) and host resistance to disease, which are elicited differently in MD-resistant and susceptible chickens103. Moreover, the potential role of γδ T cells was characterized by their significant increase in the spleen but decrease in the caecal tonsils during MDV infection, accompanied by the upregulation of IFN-γ expression in the early stage of infection and the upregulation of IL-10 expression during the late phases104,105. In addition, γδ T cells, similar to CD8+ T cells, play a critical role in early immune protection conferred by the CVI988 vaccine through differentially expressed cytotoxic and T-cell-related cytokines, except that CVI988 induces memory CD8+ T cells but not memory γδ T cells in chickens106,107. Unfortunately, persistent infection caused by MDV infection or vaccination may lead to T-cell exhaustion, which is characterized by the progressive loss of T-cell effector functions and memory properties and the upregulated expression of inhibitory receptors such as PD-1 and CTLA-4 on T cells108. Indeed, MDV infection upregulates the expression of CTLA-4, PD-1, and PD-L1 in infected chickens95,109. Furthermore, MDV infection also induces the activation of cyclooxygenase-2 (COX-2) and the production of prostaglandin E2 (PGE2), which contributes to the impairment of T-cell proliferation110.
Imperfect MD vaccination and immune failure
In most cases, MD vaccination can prevent the development of tumours but cannot block persistent MDV infection and transmission. At present, little is known about the mechanism of MD vaccine-induced immunity. Early studies suggested that vaccine-induced adaptive immunity may play a role in providing protection against MD. Multiple MDV antigens, e.g., gB, once administered as a recombinant vaccine, are immunogenic and capable of triggering immune protection. However, the types and magnitude of vaccine-induced protective immune responses to these viral antigens are still unknown. Recent studies have cast doubt on the role of T cells in MD vaccine-mediated immunity against the development of virus-induced tumours, and this doubt has made the molecular mechanism of vaccine-induced protection even more elusive111. However, compared to those in previous studies, the differences in the role of T cells in MD vaccination between distinct studies might be due to differences in immune efficacy or the mechanisms of the MD vaccines102,111,112. Moreover, several other factors, including the genetic background of chickens103, the presence or absence of maternal antibodies, the virulence of MDV epidemic strains, vaccination doses and coinfections with other immunosuppressive and neoplastic pathogens, can also affect the immune efficacy of MD vaccines113.
Since the first introduction of the MD vaccine in the 1970s, MDV has overcome the vaccine barrier through the acquisition of numerous genomic mutations, and this evolutionarily adapted virus is more lethal to chicken hosts. Widely used MD vaccines involving more virulent MDV strains have been developed, suggesting that vaccination might be a key driver of the increase in virulence114. First, MD vaccines protect chickens against the disease but still allow virus infection and transmission, which causes vvMDV epidemic strains to circulate in vaccinated chickens and potentially increases the virulence of the circulating virus by providing selective pressure115. Second, the use of live attenuated MD vaccines also has potential adverse effects, including the reversion of pathogenicity, recombination, and functional complementation in the host. Concerns about use of MD vaccine recombination to create virulent viruses have increased. In recent studies116,117, several MDV strains generated via recombination of the CVI988 backbone and the partial unique short region of the virulent MDV strain have been isolated and characterized from vaccinated chicken flocks; these strains confirm the role of live MD vaccines as genetic donors for viral genomic recombination. Complementation is another adverse mechanism associated with MD vaccines. Using natural coinfection models, the MD vaccine and MDV have been shown to infect the same cells in vivo, resulting in functional complementation, and the nontransmissible MDV was transmitted to chickens via one round of transmission118.
Due to the emergence of vv+MDV strains and HV-MDV variants, developing novel and robust next-generation MD vaccines is necessary. One attractive strategy for producing novel effective vaccine strains is to use a recombinant MDV candidate attenuated by the deletion of meq, which completely disables tumour induction and provides superior protection against MDV epidemic strains with hypervirulence. However, meq-deleted MDV mutants usually retain the ability to induce significant lymphoid organ atrophy, which has been the main obstacle for their use as vaccine candidates. A cell culture passage-attenuated meq-null MDV strain was generated to avoid this disadvantage, but this strain still showed a slight immunosuppressive effect119. In recent years, MDV mutants with double deletions of meq plus a viral early cytolytic replication gene, such as vIL8, pp38 and thymidine kinase (tk), have been generated to overcome this problem120,121,122. However, the protective efficacy of these vaccine candidates was significantly lower than that of the MDVΔMeq and CVI988/Rispens viruses. Considering that some ncRNAs, especially viral miRNAs, are also involved in the induction of lymphoid organ atrophy123,124, considering the deletion of MDV-miRNAs to reduce virus immunosuppression and improve the safety of meq-mutated MDV strains as vaccines might be worthwhile.
Discussion
Most MDV genes play important roles in DNA replication, particle formation, immunosuppression, and many other processes that are essential in the viral lifecycle. The lack of sufficient immunological techniques and key reagents makes determining the dynamic changes and functional regulation of the host immune system during MDV infection a challenge. In future studies, three pivotal tasks involved in immune regulation in response to MDV infection need to be addressed, including determining which major viral components are involved in regulating host immune responses, how MDV genes or proteins dynamically respond to the immune system to achieve immunosuppression, and how to develop a more perfect MD vaccine for disease control in the future.
Currently, a comprehensive understanding of the effector molecules expressed by MDV after virus infection or vaccination is lacking. The best characterized gene is the major oncogene meq, which is crucial for MDV pathogenesis as it not only regulates tumorigenesis but also controls the host immune response. The current “gold standard” MD vaccine strain, which is live-attenuated CVI988, expresses two meq isoforms, which are designated small meq (S-meq) with a regular size and long meq (L-meq) harbouring an 180 bp insertion in the transactivation domain. L-meq strongly enhances viral pathogenesis and tumorigenesis, while S-meq completely abrogates pathogenesis, indicating that changes in the meq gene and other mutations in the CVI988 genome may contribute to virus attenuation125. Recently, studies have shown that both gene insertion in L-meq and deletion in S-meq can enhance the transactivation of Meq, but insertion also increases the pathogenicity of MDV, while deletion reduces its pathogenicity14. Additionally, minor polymorphisms in meq acquired during evolution allow the virus to overcome innate cellular responses and vaccination-induced protection126. To better understand the molecular basis of MDV pathogenesis and enhanced virulence, further investigating the functions of viral proteins, including Meq, is necessary. In addition, MDV encodes a series of ncRNAs that are crucial for immune regulation, including miRNAs, lncRNAs, and circRNAs23. In particular, the immunoregulatory effects of MDV-encoded miRNAs have been well characterized. Considering the impact of viral miRNA-mediated inhibition on host miRNA processing when investigating MDV miRNA function is worthwhile, as such a cellular mechanism has been demonstrated in another herpesvirus, human herpesvirus 6 (HHV-6), to disrupt mitochondrial architecture, evade intrinsic host defence mechanisms, and facilitate the transition from latent to lytic infection74. With the development of new bioinformatics technologies, other types of ncRNAs, including long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs), have also been gradually identified and shown to play critical roles in MD biology23. Thus, the regulatory networks formed by ncRNAs in antiviral immunity also should be explored in the future.
Currently, the continued increase in the virulence of MDV has prompted ongoing efforts to develop more efficacious vaccines. Several techniques have been developed to selectively deliver protective antigens to APCs to enhance the protective efficacy of vaccines127. The recombinant viral vectors are also used for the development of novel poultry vaccines due to long-term antigen production and the delivery of antigens targeted with APC-specific antibodies. Several avian herpesviruses, such as HVT FC-126, infectious laryngotracheitis virus (ILTV) and some MDV-1 strains, have been genetically modified to be vectors for vaccines combating multiple avian diseases128. Recently, the use of extracellular vesicles (EVs) has emerged as a novel strategy for developing animal vaccines129. EVs have a potential role in the activation of cellular and antibody immune responses in MDV infection, thus opening up the possibility of developing a new MDV vaccination platform130. Considering the increasing number of important roles of the innate immune system in protection mediated by vaccination, the protective capacity of MD vaccines would greatly benefit from the development of new recombinant vaccines or adjuvants that enhance immune activation. More biological techniques, especially the generation of CRISPR/Cas9-mediated genome editing technologies131, must be channelled into the practical generation of novel, efficacious, safe, and broadly protective vaccines.
Considering that the currently used MD vaccines cannot prevent the infection and spread of MDV epidemic strains, additional alternative strategies for the efficient control of this disease are needed. In a recent study132, the sulfated polysaccharide extract ulvans from Ulva armoricana was verified to exert antiviral activity by limiting the replication/dissemination of viruses or stimulating the innate immune system of hosts, which may be an alternative to drug therapy for MDV infection. A study on the differential expression of purinergic receptors (PRs) in response to MDV infection and disease progression has demonstrated their crucial role in the process of disease; PRs may provide another potential target for MD therapy133. The generation of breeding chicken lines with more robust immune resistance to MDV may be another effective strategy to address the current epidemic and emerging viruses. Transgenic chickens stably expressing Cas9 and gRNAs to target the viral ICP4 gene for intracellular defence against MDV were recently generated to increase resistance to MD134. Furthermore, studies on the genetic variation in IFN signalling pathway genes may provide more possibilities for enhancing resistance to MD in chickens135.
In summary, we have reviewed the known strategies by which MDV evades immune surveillance mediated by distinct subsets of the host immune system. With the recent advancements in biotechnology and the application of new approaches in virology, more findings about the cellular immune response to MDV infection and/or vaccination will be uncovered in the near future. All of this meaningful progress will facilitate our work on the development of the next generation of efficient MD vaccines.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or in the materials cited herein. Additional data related to this paper may be requested from the authors.
References
Nair, V. et al. in Diseases of Poultry (ed Martine Boulianne David E. Swayne, Catherine M. Logue, Larry R. McDougald, Venugopal Nair, David L. Suarez, Sjaak de Wit, Tom Grimes, Deirdre Johnson, Michelle Kromm, Teguh Yodiantara Prajitno, Ian Rubinoff, Guillermo Zavala) 548-715 (John Wiley & Sons Inc, 2020).
Gatherer, D. et al. ICTV Virus Taxonomy Profile: Herpesviridae 2021. J. Gen. Virol. 102, 001673 (2021).
Witter, R. L. Increased virulence of Marek’s disease virus field isolates. Avian Dis. 41, 149–163 (1997).
Reddy, S. M., Izumiya, Y. & Lupiani, B. Marek’s disease vaccines: Current status, and strategies for improvement and development of vector vaccines. Vet. Microbiol. 206, 113–120 (2017).
Liu, J. L. et al. Emerging Hypervirulent Marek’s Disease Virus Variants Significantly Overcome Protection Conferred by Commercial Vaccines. Viruses 15, 1434 (2023).
Ghalyanchilangeroudi, A. et al. Molecular Characterization and Phylogenetic Analysis of Marek’s Disease Virus in Iran. Avian Dis. 66, 1–5 (2022).
Adedeji, A., Abdu, P., Akanbi, O. & Luka, P. Molecular and pathological investigations of Marek’s disease outbreaks in vaccinated poultry farms in Plateau State, North Central-Nigeria. Veterinaria Ital. 58, 77–85 (2022).
Wannaratana, S., Tunterak, W., Prakairungnamthip, D., Sasipreeyajan, J. & Thontiravong, A. Genetic characterization of Marek’s disease virus in chickens in Thailand reveals a high genetic diversity of circulating strains. Transbound. Emerg. Dis. 69, 3771–3779 (2022).
Song, B. & Zeb, J. A Review on the Marek’s Disease Outbreak and Its Virulence-Related meq Genovariation in Asia between 2011 and 2021. Animals 12, 540 (2022).
Schat, K. A. History of the First-Generation Marek’s Disease Vaccines: The Science and Little-Known Facts. Avian Dis. 60, 715–724 (2016).
Dunn, J. R., Black Pyrkosz, A., Steep, A. & Cheng, H. H. Identification of Marek’s disease virus genes associated with virulence of US strains. J. Gen. Virol. 100, 1132–1139 (2019).
Deng, Q., Shi, M., Li, Q. & Wang, P. Analysis of the evolution and transmission dynamics of the field MDV in China during the years 1995-2020, indicating the emergence of a unique cluster with the molecular characteristics of vv+ MDV that has become endemic in southern China. Transbound. Emerg. Dis. 68, 3574–3587 (2021).
Yehia, N., El-Sayed, H. S., Omar, S. E., Erfan, A. & Amer, F. Genetic evolution of Marek’s disease virus in vaccinated poultry farms. Vet. World 14, 1342–1353 (2021).
Sato, J. et al. Effect of Insertion and Deletion in the Meq Protein Encoded by Highly Oncogenic Marek’s Disease Virus on Transactivation Activity and Virulence. Viruses 14, 382 (2022).
Teng, M. et al. Pathogenicity and Pathotype Analysis of Henan Isolates of Marek’s Disease Virus Reveal Long-Term Circulation of Highly Virulent MDV Variant in China. Viruses 14, 1651 (2022).
Barrow, A. D., Burgess, S. C., Baigent, S. J., Howes, K. & Nair, V. K. Infection of macrophages by a lymphotropic herpesvirus: a new tropism for Marek’s disease virus. J. Gen. Virol. 84, 2635–2645 (2003).
Berthault, C. et al. Atrophy of primary lymphoid organs induced by Marek’s disease virus during early infection is associated with increased apoptosis, inhibition of cell proliferation and a severe B-lymphopenia. Vet. Res. 49, 31 (2018).
Baigent, S. J. & Davison, F. 6 - Marek’s disease virus: Biology and life cycle. (eds Fred Davison & Venugopal Nair) 62-77, i-ii (Academic Press, 2004).
Hunt, H. D. et al. Marek’s disease virus down-regulates surface expression of MHC (B Complex) Class I (BF) glycoproteins during active but not latent infection of chicken cells. Virology 282, 198–205 (2001).
Li, K. et al. Avian oncogenic herpesvirus antagonizes the cGAS-STING DNA-sensing pathway to mediate immune evasion. PLoS Pathog. 15, e1007999 (2019).
Dong, K., Chang, S., Xie, Q., Zhao, P. & Zhang, H. RNA Sequencing revealed differentially expressed genes functionally associated with immunity and tumor suppression during latent phase infection of a vv + MDV in chickens. Sci. Rep. 9, 14182 (2019).
Bertzbach, L. D., Kheimar, A., Ali, F. A. Z. & Kaufer, B. B. Viral Factors Involved in Marek’s Disease Virus (MDV) Pathogenesis. Curr. Clin. Microbiol. Rep. 5, 238–244 (2018).
Teng, M. et al. Critical roles of non-coding RNAs in lifecycle and biology of Marek’s disease herpesvirus. Sci. China Life Sci. 66, 251–268 (2023).
Chakraborty, P., Kuo, R. & Vervelde, L. Macrophages from Susceptible and Resistant Chicken Lines have Different Transcriptomes following Marek’s Disease Virus Infection. Genes 10, 74 (2019).
Chakraborty, P. et al. The Role of Dendritic Cells in the Host Response to Marek’s Disease Virus (MDV) as Shown by Transcriptomic Analysis of Susceptible and Resistant Birds. Pathog. (Basel, Switz.) 11, 1340 (2022).
Sun, G. R. et al. Differential expression of type I interferon mRNA and protein levels induced by virulent Marek’s disease virus infection in chickens. Vet. Immunol. Immunopathol. 212, 15–22 (2019).
Jin, H. et al. Transcriptional Profiles Associated with Marek’s Disease Virus in Bursa and Spleen Lymphocytes Reveal Contrasting Immune Responses during Early Cytolytic Infection. Viruses 12, 354 (2020).
Jin, Y. C. et al. Study on the contrast of the MHC-peptide interaction of B2/B21 haplotype and MHC-related virus resistance in chickens. Immun. Inflamm. Dis. 9, 1670–1677 (2021).
Hearn, C., Preeyanon, L., Hunt, H. D. & York, I. A. An MHC class I immune evasion gene of Marek׳s disease virus. Virology 475, 88–95 (2015).
Jarosinski, K. W., Hunt, H. D. & Osterrieder, N. Down-regulation of MHC class I by the Marek’s disease virus (MDV) UL49.5 gene product mildly affects virulence in a haplotype-specific fashion. Virology 405, 457–463 (2010).
Halabi, S. & Ghosh, M. The dominantly expressed class II molecule from a resistant MHC haplotype presents only a few Marek’s disease virus peptides by using an unprecedented binding motif. PLoS Biol. 19, e3001057 (2021).
Niikura, M. et al. Marek’s disease virus up-regulates major histocompatibility complex class II cell surface expression in infected cells. Virology 359, 212–219 (2007).
Thanthrige-Don, N. et al. Marek’s disease virus influences the expression of genes associated with IFN-gamma-inducible MHC class II expression. Viral Immunol. 23, 227–232 (2010).
Levy, A. M. et al. Marek’s disease virus Meq transforms chicken cells via the v-Jun transcriptional cascade: A converging transforming pathway for avian oncoviruses. Proc. Natl Acad. Sci. 102, 14831–14836 (2005).
Levy, A. M. et al. Characterization of the chromosomal binding sites and dimerization partners of the viral oncoprotein Meq in Marek’s disease virus-transformed T cells. J. Virol. 77, 12841–12851 (2003).
Subramaniam, S. et al. Integrated analyses of genome-wide DNA occupancy and expression profiling identify key genes and pathways involved in cellular transformation by a Marek’s disease virus oncoprotein, Meq. J. Virol. 87, 9016–9029 (2013).
Osterrieder, N., Kamil, J. P., Schumacher, D., Tischer, B. K. & Trapp, S. Marek’s disease virus: from miasma to model. Nat. Rev. Microbiol. 4, 283–294 (2006).
Boodhoo, N., Gurung, A., Sharif, S. & Behboudi, S. Marek’s disease in chickens: a review with focus on immunology. Vet. Res. 47, 119 (2016).
Strassheim, S., Stik, G., Rasschaert, D. & Laurent, S. mdv1-miR-M7-5p, located in the newly identified first intron of the latency-associated transcript of Marek’s disease virus, targets the immediate-early genes ICP4 and ICP27. J. Gen. Virol. 93, 1731–1742 (2012).
Boumart, I. et al. GaHV-2 ICP22 protein is expressed from a bicistronic transcript regulated by three GaHV-2 microRNAs. J. Gen. Virol. 99, 1286–1300 (2018).
Zhuang, G., Sun, A., Teng, M. & Luo, J. A Tiny RNA that Packs a Big Punch: The Critical Role of a Viral miR-155 Ortholog in Lymphomagenesis in Marek’s Disease. Front. Microbiol. 8, 1169 (2017).
Muylkens, B., Coupeau, D., Dambrine, G., Trapp, S. & Rasschaert, D. Marek’s disease virus microRNA designated Mdv1-pre-miR-M4 targets both cellular and viral genes. Arch. Virol. 155, 1823–1837 (2010).
Zhou, L. & Zheng, S. J. The Roles of MicroRNAs (miRNAs) in Avian Response to Viral Infection and Pathogenesis of Avian Immunosuppressive Diseases. Int. J. Mol. Sci. 20, 5454 (2019).
Steep, A. et al. Identification and Validation of Ikaros (IKZF1) as a Cancer Driver Gene for Marek’s Disease Virus-Induced Lymphomas. Microorganisms 10, 401 (2022).
Iempridee, T. et al. Epstein-Barr virus utilizes Ikaros in regulating its latent-lytic switch in B cells. J. Virol. 88, 4811–4827 (2014).
Robinson, C. M., Hunt, H. D., Cheng, H. H. & Delany, M. E. Chromosomal integration of an avian oncogenic herpesvirus reveals telomeric preferences and evidence for lymphoma clonality. Herpesviridae 1, 5 (2010).
Osterrieder, N., Wallaschek, N. & Kaufer, B. B. Herpesvirus Genome Integration into Telomeric Repeats of Host Cell Chromosomes. Annu. Rev. Virol. 1, 215–235 (2014).
Kaufer, B. B., Jarosinski, K. W. & Osterrieder, N. Herpesvirus telomeric repeats facilitate genomic integration into host telomeres and mobilization of viral DNA during reactivation. J. Exp. Med. 208, 605–615 (2011).
Greco, A., Fester, N., Engel, A. T. & Kaufer, B. B. Role of the short telomeric repeat region in Marek’s disease virus replication, genomic integration, and lymphomagenesis. J. Virol. 88, 14138–14147 (2014).
Pejaković, S. et al. Role of DNA Methylation and CpG Sites in the Viral Telomerase RNA Promoter during Gallid Herpesvirus 2 Pathogenesis. J. Virol. 94, e01488–01420 (2020).
Shkreli, M., Dambrine, G., Soubieux, D., Kut, E. & Rasschaert, D. Involvement of the oncoprotein c-Myc in viral telomerase RNA gene regulation during Marek’s disease virus-induced lymphomagenesis. J. Virol. 81, 4848–4857 (2007).
Robinson, C. M., Cheng, H. H. & Delany, M. E. Temporal kinetics of Marek’s disease herpesvirus: integration occurs early after infection in both B and T cells. Cytogenetic Genome Res. 144, 142–154 (2014).
Chi, J. Q. et al. Marek’s disease virus-encoded analog of microRNA-155 activates the oncogene c-Myc by targeting LTBP1 and suppressing the TGF-beta signaling pathway. Virology 476, 72–84 (2015).
Bencherit, D. et al. Induction of DNA Damages upon Marek’s Disease Virus Infection: Implication in Viral Replication and Pathogenesis. J. Virol. 91, e01658–01617 (2017).
Jha, H. C. et al. H2AX phosphorylation is important for LANA-mediated Kaposi’s sarcoma-associated herpesvirus episome persistence. J. Virol. 87, 5255–5269 (2013).
You, Y. et al. A Cell Culture System to Investigate Marek’s Disease Virus Integration into Host Chromosomes. Microorganisms 9, 2489 (2021).
Gimeno, I. M. & Schat, K. A. Virus-Induced Immunosuppression in Chickens. Avian Dis. 62, 272–285 (2018).
Bertzbach, L. D. et al. IFNalpha and IFNgamma Impede Marek’s Disease Progression. Viruses 11, 1103 (2019).
Feng, Z. Q., Lian, T., Huang, Y., Zhu, Q. & Liu, Y. P. Expression pattern of genes of RLR-mediated antiviral pathway in different-breed chicken response to Marek’s disease virus infection. Biomed. Res. Int. 2013, 419256 (2013).
Liu, Y. et al. Marek’s Disease Virus RLORF4 Inhibits Type I Interferon Production by Antagonizing NF-kappaB Activation. J. Virol. 93, e01037–01019 (2019).
Gao, L. et al. Inhibition of DNA-Sensing Pathway by Marek’s Disease Virus VP23 Protein through Suppression of Interferon Regulatory Factor 7 Activation. J. Virol. 93, e01934–01918 (2019).
Du, X. et al. Marek’s disease virus serine/threonine kinase Us3 facilitates viral replication by targeting IRF7 to block IFN-beta production. Vet. Microbiol. 266, 109364 (2022).
Liao, Y. et al. Role of Marek’s Disease Virus (MDV)-Encoded US3 Serine/Threonine Protein Kinase in Regulating MDV Meq and Cellular CREB Phosphorylation. J. Virol. 94, e00892–00820 (2020).
Zou, H. et al. Toll-like receptor 3 pathway restricts Marek’s disease virus infection. Oncotarget 8, 70847–70853 (2017).
Bavananthasivam, J., Kulkarni, R. R., Read, L. & Sharif, S. Reduction of Marek’s Disease Virus Infection by Toll-Like Receptor Ligands in Chicken Embryo Fibroblast Cells. Viral Immunol. 31, 389–396 (2018).
Xu, J. et al. DEAD/DEAH-box helicase 5 is hijacked by an avian oncogenic herpesvirus to inhibit interferon beta production and promote viral replication. Developmental Comp. Immunol. 119, 104048 (2021).
Figueroa, T., Boumart, I., Coupeau, D. & Rasschaert, D. Hyperediting by ADAR1 of a new herpesvirus lncRNA during the lytic phase of the oncogenic Marek’s disease virus. J. Gen. Virol. 97, 2973–2988 (2016).
Li, Z., Okonski, K. M. & Samuel, C. E. Adenosine deaminase acting on RNA 1 (ADAR1) suppresses the induction of interferon by measles virus. J. Virol. 86, 3787–3794 (2012).
Mannion, N. M. et al. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep. 9, 1482–1494 (2014).
Hu, X. et al. Activation of Toll-like receptor 3 inhibits Marek’s disease virus infection in chicken embryo fibroblast cells. Arch. Virol. 161, 521–528 (2016).
Parnas, O., Corcoran, D. L. & Cullen, B. R. Analysis of the mRNA targetome of microRNAs expressed by Marek’s disease virus. mBio 5, e01060–01013 (2014).
Lu, F. et al. Epstein-Barr virus-induced miR-155 attenuates NF-kappaB signaling and stabilizes latent virus persistence. J. Virol. 82, 10436–10443 (2008).
Bondada, M. S., Yao, Y. & Nair, V. Multifunctional miR-155 Pathway in Avian Oncogenic Virus-Induced Neoplastic Diseases. Noncoding RNA 5, 24 (2019).
Hennig, T. et al. Selective inhibition of miRNA processing by a herpesvirus-encoded miRNA. Nature 605, 539–544 (2022).
Wang, D., Sun, S. & Heidari, M. Marek’s disease vaccine activates chicken macrophages. J. Vet. Sci. 19, 375–383 (2018).
Fan, Z., Wang, H., Pan, J., Yu, S. & Xia, W. Potential Role of Macrophage Migration Inhibitory Factor in the Pathogenesis of Marek’s Disease. J. Vet. Res. 64, 33–38 (2020).
Mitchell, R. A. et al. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc. Natl Acad. Sci. USA 99, 345–350 (2002).
Nobre, C. C. et al. Macrophage Migration Inhibitory Factor (MIF): Biological Activities and Relation with Cancer. Pathol. Oncol. Res. 23, 235–244 (2017).
Ostuni, R., Kratochvill, F., Murray, P. J. & Natoli, G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 36, 229–239 (2015).
Grauwet, K. et al. Modulation of CD112 by the alphaherpesvirus gD protein suppresses DNAM-1-dependent NK cell-mediated lysis of infected cells. Proc. Natl Acad. Sci. USA 111, 16118–16123 (2014).
Campbell, T. M., McSharry, B. P., Steain, M., Slobedman, B. & Abendroth, A. Varicella-Zoster Virus and Herpes Simplex Virus 1 Differentially Modulate NKG2D Ligand Expression during Productive Infection. J. Virol. 89, 7932–7943 (2015).
Kim, T., Hunt, H. D., Parcells, M. S., van Santen, V. & Ewald, S. J. Two class I genes of the chicken MHC have different functions: BF1 is recognized by NK cells while BF2 is recognized by CTLs. Immunogenetics 70, 599–611 (2018).
Bertzbach, L. D., van Haarlem, D. A., Härtle, S., Kaufer, B. B. & Jansen, C. A. Marek’s Disease Virus Infection of Natural Killer Cells. Microorganisms 7, 588 (2019).
Spelbrink, J. N. Functional organization of mammalian mitochondrial DNA in nucleoids: history, recent developments, and future challenges. IUBMB life 62, 19–32 (2010).
West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015).
Chodkowski, M. et al. Human herpesvirus type 1 and type 2 disrupt mitochondrial dynamics in human keratinocytes. Arch. Virol. 163, 2663–2673 (2018).
Reddy, V., Sadigh, Y., Tang, N., Yao, Y. & Nair, V. Novel Insights into the Roles of Bcl-2 Homolog Nr-13 (vNr-13) Encoded by Herpesvirus of Turkeys in the Virus Replication Cycle, Mitochondrial Networks, and Apoptosis Inhibition. J. Virol. 94, e02049–02019 (2020).
Chu, Q., Ding, Y., Cai, W., Liu, L. & Zhang, H. Marek’s Disease Virus Infection Induced Mitochondria Changes in Chickens. Int. J. Mol. Sci. 20, 3150 (2019).
Umthong, S., Dunn, J. R. & Cheng, H. H. Towards a mechanistic understanding of the synergistic response induced by bivalent Marek’s disease vaccines to prevent lymphomas. Vaccine 37, 6397–6404 (2019).
Heidari, M., Zhang, H., Hearn, C. & Sunkara, L. B cells do not play a role in vaccine-mediated immunity against Marek’s disease. Vaccine: X 10, 100128 (2022).
Bertzbach, L. D., Laparidou, M. & Härtle, S. Unraveling the role of B cells in the pathogenesis of an oncogenic avian herpesvirus. Proc. Natl Acad. Sci. USA 115, 11603–11607 (2018).
Trapp-Fragnet, L. & Schermuly, J. Marek’s disease virus prolongs survival of primary chicken B-cells by inducing a senescence-like phenotype. PLoS Pathog. 17, e1010006 (2021).
Giles, J. R., Kashgarian, M., Koni, P. A. & Shlomchik, M. J. B. Cell-Specific MHC Class II Deletion Reveals Multiple Nonredundant Roles for B Cell Antigen Presentation in Murine Lupus. J. Immunol. (Baltim., Md. : 1950) 195, 2571–2579 (2015).
Yang, Y., Dong, M., Hao, X., Qin, A. & Shang, S. Revisiting cellular immune response to oncogenic Marek’s disease virus: the rising of avian T-cell immunity. Cell. Mol. Life Sci. 77, 3103–3116 (2020).
Parvizi, P., Andrzejewski, K., Read, L. R., Behboudi, S. & Sharif, S. Expression profiling of genes associated with regulatory functions of T-cell subsets in Marek’s disease virus-infected chickens. Avian Pathol. : J. W. V. P. A 39, 367–373 (2010).
Okazaki, T., Chikuma, S., Iwai, Y., Fagarasan, S. & Honjo, T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 14, 1212–1218 (2013).
Zhou, X. et al. Marek’s Disease Virus Regulates the Ubiquitylome of Chicken CD4(+) T Cells to Promote Tumorigenesis. Int. J. Mol. Sci. 20, 2089 (2019).
Parvizi, P. et al. Cytokine gene expression in splenic CD4+ and CD8+ T cell subsets of genetically resistant and susceptible chickens infected with Marek’s disease virus. Vet. Immunol. Immunopathol. 132, 209–217 (2009).
Engel, A. T., Selvaraj, R. K., Kamil, J. P., Osterrieder, N. & Kaufer, B. B. Marek’s disease viral interleukin-8 promotes lymphoma formation through targeted recruitment of B cells and CD4+ CD25+ T cells. J. Virol. 86, 8536–8545 (2012).
Gurung, A. & Kamble, N. Association of Marek’s Disease induced immunosuppression with activation of a novel regulatory T cells in chickens. PLoS Pathog. 13, e1006745 (2017).
Bavananthasivam, J. et al. The Regulatory Microenvironment in Feathers of Chickens Infected with Very Virulent Marek’s Disease Virus. Viruses 14, 112 (2022).
Umthong, S. & Dunn, J. R. Depletion of CD8αβ(+) T Cells in Chickens Demonstrates Their Involvement in Protective Immunity towards Marek’s Disease with Respect to Tumor Incidence and Vaccinal Protection. Vaccines 8, 557 (2020).
Boodhoo, N. & Behboudi, S. Differential Virus-Specific IFN-Gamma Producing T Cell Responses to Marek’s Disease Virus in Chickens With B19 and B21 MHC Haplotypes. Front. Immunol. 12, 784359 (2021).
Laursen, A. M. S. et al. Characterizaton of gamma delta T cells in Marek’s disease virus (Gallid herpesvirus 2) infection of chickens. Virology 522, 56–64 (2018).
Matsuyama-Kato, A. et al. Activated Chicken Gamma Delta T Cells Are Involved in Protective Immunity against Marek’s Disease. Viruses 15, 285 (2023).
Hao, X. et al. An Anti-Tumor Vaccine Against Marek’s Disease Virus Induces Differential Activation and Memory Response of γδ T Cells and CD8 T Cells in Chickens. Front. Immunol. 12, 645426 (2021).
Matsuyama-Kato, A. et al. Phenotypic characterization of gamma delta (γδ) T cells in chickens infected with or vaccinated against Marek’s disease virus. Virology 568, 115–125 (2022).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Matsuyama-Kato, A. et al. Molecular characterization of immunoinhibitory factors PD-1/PD-L1 in chickens infected with Marek’s disease virus. Virol. J. 9, 94 (2012).
Kamble, N., Gurung, A., Kaufer, B. B., Pathan, A. A. & Behboudi, S. Marek’s Disease Virus Modulates T Cell Proliferation via Activation of Cyclooxygenase 2-Dependent Prostaglandin E2. Front. Immunol. 12, 801781 (2021).
Heidari, M. & Zhang, H. Role of T Cells in Vaccine-Mediated Immunity against Marek’s Disease. Viruses 15, 648 (2023).
Morimura, T. et al. Anti-viral and anti-tumor effects induced by an attenuated Marek’s disease virus in CD4- or CD8-deficient chickens. Arch. Virol. 144, 1809–1818 (1999).
Wang, P. et al. Vertical transmission of ALV from ALV-J positive parents caused severe immunosuppression and significantly reduced marek’s disease vaccine efficacy in three-yellow chickens. Vet. Microbiol. 244, 108683 (2020).
Li, H., Ge, Z., Luo, Q., Fu, Q. & Chen, R. A highly pathogenic Marek’s disease virus isolate from chickens immunized with a bivalent vaccine in China. Arch. Virol. 167, 861–870 (2022).
Read, A. F. et al. Imperfect Vaccination Can Enhance the Transmission of Highly Virulent Pathogens. PLoS Biol. 13, e1002198 (2015).
He, L. et al. Genomic analysis of a Chinese MDV strain derived from vaccine strain CVI988 through recombination. Infect., Genet. evolution: J. Mol. Epidemiol. Evolut. Genet. Infect. Dis. 78, 104045 (2020).
Zhang, Y. et al. Emerging natural recombinant Marek’s disease virus between vaccine and virulence strains and their pathogenicity. Transbound. Emerg. Dis. 69, e1702–e1709 (2022).
Xu, H. & Krieter, A. L. Coinfection in the host can result in functional complementation between live vaccines and virulent virus. Virulence 13, 980–989 (2022).
Sun, P. et al. Attenuation of a recombinant Marek’s disease virus lacking the meq oncogene and evaluation on its immune efficacy against Marek’s disease virus. Poult. Sci. 99, 1939–1945 (2020).
Liao, Y., Reddy, S. M. & Khan, O. A. A Novel Effective and Safe Vaccine for Prevention of Marek’s Disease Caused by Infection with a Very Virulent Plus (vv+) Marek’s Disease Virus. Vaccines 9, 159 (2021).
Sun, A. et al. Fully Attenuated meq and pp38 Double Gene Deletion Mutant Virus Confers Superior Immunological Protection against Highly Virulent Marek’s Disease Virus Infection. Microbiology spectrum 10, e0287122 (2022).
Conrad, S. J. & Oluwayinka, E. B. Deletion of the Viral Thymidine Kinase in a Meq-Deleted Recombinant Marek’s Disease Virus Reduces Lymphoid Atrophy but Is Less Protective. Microorganisms 10, 7 (2021).
Bai, Y. et al. Deletion of miR-M8 and miR-M13 eliminates the bursa atrophy induced by Marek’s disease virus infection. Vet. Microbiol. 268, 109409 (2022).
Teng, M. et al. The significance of the individual Meq-clustered miRNAs of Marek’s disease virus in oncogenesis. J. Gen. Virol. 96, 637–649 (2015).
Conradie, A. M. & Bertzbach, L. D. A common live-attenuated avian herpesvirus vaccine expresses a very potent oncogene. mSphere 4, e00658–00619 (2019).
Conradie, A. M. & Bertzbach, L. D. Distinct polymorphisms in a single herpesvirus gene are capable of enhancing virulence and mediating vaccinal resistance. PLoS Pathog. 16, e1009104 (2020).
Shrestha, A., Sadeyen, J. R. & Iqbal, M. Enhancing Protective Efficacy of Poultry Vaccines through Targeted Delivery of Antigens to Antigen-Presenting Cells. Vaccines 6, 75 (2018).
Baron, M. D., Iqbal, M. & Nair, V. Recent advances in viral vectors in veterinary vaccinology. Curr. Opin. Virol. 29, 1–7 (2018).
Negahdaripour, M., Vakili, B. & Nezafat, N. Exosome-based vaccines and their position in next generation vaccines. Int. Immunopharmacol. 113, 109265 (2022).
Neerukonda, S. N., Tavlarides-Hontz, P., McCarthy, F., Pendarvis, K. & Parcells, M. S. Comparison of the Transcriptomes and Proteomes of Serum Exosomes from Marek’s Disease Virus-Vaccinated and Protected and Lymphoma-Bearing Chickens. Genes 10, 116 (2019).
Teng, M., Yao, Y. & Nair, V. Latest Advances of Virology Research Using CRISPR/Cas9-Based Gene-Editing Technology and Its Application to Vaccine Development. Viruses 13, 779 (2021).
Bussy, F., Rémy, S., Le Goff, M., Collén, P. N. & Trapp-Fragnet, L. The sulphated polysaccharides extract ulvans from Ulva armoricana limits Marek’s disease virus dissemination in vitro and promotes viral reactivation in lymphoid cells. BMC Vet. Res. 18, 155 (2022).
Akbar, H., Fasick, J. J., Ponnuraj, N. & Jarosinski, K. W. Purinergic signaling during Marek’s disease in chickens. Sci. Rep. 13, 2044 (2023).
Challagulla, A., Jenkins, K. A., O’Neil, T. E., Shi, S. & Morris, K. R. In Vivo Inhibition of Marek’s Disease Virus in Transgenic Chickens Expressing Cas9 and gRNA against ICP4. Microorganisms 9, 164 (2021).
Mountford, J., Gheyas, A., Vervelde, L. & Smith, J. Genetic variation in chicken interferon signalling pathway genes in research lines showing differential viral resistance. Anim. Genet. 53, 640–656 (2022).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (no. U21A20260), the Natural Science Foundation for Distinguished Young Scholars of Henan Province (no. 232300421009), the Key Scientific Research Projects of Universities in Henan supported by the Education Department of Henan Province (no. 22A310017), the Scientific and Technological Research Project Foundation of Henan Provincial Scientific and Technological Department (no. 202102310479, no. 222102110180), and the Biotechnology and Biological Sciences Research Council (BBSRC) (grant numbers BB/R012865/1 and BBS/OS/NW/000007).
Author information
Authors and Affiliations
Contributions
Z.J.Z., M.T., Y.L., and F.J.C. performed the data collection, discussion and wrote the draft of manuscript. Z.J.Z. drew the figures. Z.J.Z., Y.Y., E.Z.L., and J.L. revised the paper. Z.J.Z., M.T., and J.L. provided additional text and edits.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, ZJ., Teng, M., Liu, Y. et al. Immune escape of avian oncogenic Marek’s disease herpesvirus and antagonistic host immune responses. npj Vaccines 9, 109 (2024). https://doi.org/10.1038/s41541-024-00905-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-024-00905-0
- Springer Nature Limited