Abstract
Screening for polycystic ovary syndrome (PCOS) in antenatal care is inadequate, largely owing to the lack of clarity around whether PCOS is an independent risk factor for pregnancy complications. This systematic review and meta-analysis include 104 studies and 106,690 pregnancies in women with and without PCOS from inception until 13th July 2022. We report that women with PCOS are younger and have higher body mass index (BMI) around conception and have greater gestational weight gain. The odds of miscarriage, gestational diabetes mellitus, gestational hypertension, pre-eclampsia and cesarean section are higher in women with PCOS. The increased odds of adverse outcomes in PCOS remain significant when age and BMI are matched and when analyses are restricted to high-quality studies. This work informed the recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome, emphasizing that PCOS status should be captured in all women who are planning to, or have recently become pregnant to facilitate prevention of adverse outcomes and improve pregnancy outcomes.
Similar content being viewed by others
Introduction
Polycystic ovary syndrome (PCOS) is a lifelong condition and, with a prevalence of 5% to 13%, is the most prevalent endocrinopathy in women of reproductive age1. In adults, diagnosis is based on a minimum of two criteria of hyperandrogenism, oligo-anovulation and/or polycystic ovaries2. Obesity is common in PCOS3 and exacerbates intrinsic insulin resistance and the clinical manifestations of PCOS4. Anxiety, depression and poor quality of life are also more common in women with PCOS compared with women without PCOS5. Affected women are also more likely to have anovulatory infertility6, requiring treatments such as assisted reproductive technology (ART) to conceive. While each of the metabolic, psychological, and reproductive features of PCOS is recognized in antenatal care guidelines as an independent risk factor for pregnancy complications, women with PCOS are not universally considered a high-risk group for pregnancy complications7.
In previous meta-analyses, women with PCOS were shown to have elevated odds of pregnancy complications. These include significantly higher odds of miscarriage, gestational diabetes mellitus (GDM), gestational hypertension, pre-eclampsia, cesarean section8,9,10,11,12, gestational weight gain8, and induction of labor8, compared with women without PCOS. Instrumental delivery was similar in women with and without PCOS9,10, while perinatal depression was an untapped area of research in this population8. Importantly, the increased odds of pregnancy complications in PCOS were influenced by PCOS diagnostic criteria, age, obesity, conception mode8, and quality of studies8,9,11.
While empirical evidence demonstrates that women with PCOS have higher odds of pregnancy complications, substantial clinical heterogeneity was reported in pooled analyses. Furthermore, subgroups and univariate or multivariate meta‐regression analyses had small numbers of studies, which were insufficient to obtain meaningful results8. In the context of the 2023 update of the International Evidence‐Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome2, we aimed to update our prior systematic review, meta-analysis, and meta‐regression8 to determine the prevalence of pregnancy complications in women with and without PCOS. With an expanded data set, we also aimed to explore whether pregnancy outcomes in women with and without PCOS are affected by age, body mass index (BMI), conception with ART, or high-quality study design.
Results
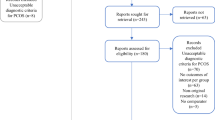
The literature search identified 4595 articles published since 2017, of which 39 studies were included in the meta-analysis after full-text review. Agreements between the reviewers in the title and abstract screening and full-text screening were considered good and excellent, with kappa values of 0.72 and 0.94, respectively13. There were 77 studies in the 2017 meta-analysis, of which 65 studies were included (after removing those not meeting the updated eligibility criteria for PCOS diagnosis). Combining the new and previous articles, a total of 104 articles were included in the present systematic review (Fig. 1).
The outcomes were reported in 17384 women with PCOS and 89,306 women without PCOS (Supplementary Table 1). Fifty-two studies were conducted in Asia, 26 in Europe, 21 in America, two in Australia and New Zealand and two in Africa. One study recruited women with multiple pregnancies14. Six studies reported outcomes in women who took metformin after conception15,16,17,18,19,20, and one study reported outcomes in women who had conceived after bariatric surgery21. Thirty-three studies reported outcomes in post-ART pregnancies22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54, and four reported outcomes in pregnancies with GDM in women with and without PCOS55,56,57,58. Sixteen studies had a high-quality design18,25,36,48,56,59,60,61,62,63,64,65,66,67,68,69. Twenty-seven studies matched women with and without PCOS for age17,18,19,22,32,36,48,56,59,60,61,62,63,64,66,67,68,69,70,71,72,73,74,75,76,77,78 and 14 for age and BMI36,48,56,60,61,62,63,64,66,68,70,75,77,78. No publication bias was found for any of the outcomes. The certainty of evidence for the outcomes was very low to moderate, mainly due to a high risk of bias, serious inconsistency, and serious indirectness. Further details are delineated in the Technical Report for the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome79.
Overall, 69 studies reported age, and 58 studies reported BMI either at preconception or at early pregnancy. Women with PCOS were younger (MD: −0.63 years; 95% CI: −0.87, −0.39) and had a higher BMI (1.76 kg/m2; 1.44, 2.08) compared with women without PCOS. Sensitivity analysis showed that after exclusion of studies in which women were taking metformin after conception16,17,20 or conceived after bariatric surgery21, lower age (−0.68 years; −0.93, −0.44) and higher BMI (1.68 kg/m2; 1.35, 2.00) remained significant in PCOS. Table 1 shows the effect sizes for outcomes of interest on pooled and sensitivity analyses. Forest and cumulative plots, funnel plots (Supplementary Figs. 1a to 10a) and Egger’s test results are provided in the Supplementary information.
Gestational weight gain
Sixteen studies reported mean gestational weight gain in 755 women with PCOS and 1765 women without PCOS41,56,58,59,64,71,74,75,77,80,81,82,83,84,85,86. Women with PCOS had higher gestational weight gain compared with women without PCOS (MD: 0.96 kg; 95% CI: 0.01, 1.90). There were no studies in which women were taking metformin after conception. Cumulative meta-analysis suggested that the MD of gestational weight gain in women with and without PCOS did not substantially change over time; however, studies published before 1999 (n = 2) contributed to heterogeneity. Higher gestational weight gain was not retained in post-ART pregnancies41, prospective56,58,75,83,84,85 and high-quality studies56,59,64. Two studies reported gestational weight gain in pregnancies with GDM56,58; gestational weight gain was higher in women with PCOS (1.81 kg; 0.42, 3.19). In seven studies, women with and without PCOS were matched for age56,59,64,71,74,75,77 and in four for both age and BMI56,64,75,77; PCOS was associated with gestational weight gain in neither age-matched nor age- and BMI-matched studies.
Miscarriage
Forty-five studies reported miscarriage20,21,22,23,24,25,26,27,28,29,30,31,34,35,36,37,38,39,42,43,44,45,46,47,48,49,50,51,52,54,62,63,65,66,77,78,87,88,89,90,91,92,93,94,95; one study was excluded from the meta-analysis because of overlapping participants62, leaving 9893 women with PCOS and 57411 women without PCOS. Miscarriage was defined as pregnancy loss within the first six to eight weeks of pregnancy (using serial ultrasound) in one study34 or within the first 12 weeks of pregnancy in seven studies36,38,40,54,88,91,93, birth prior to 20 weeks of pregnancy in two studies38,77, spontaneous pregnancy loss prior to 20 weeks of pregnancy in three studies24,25,43, prior to 24 weeks of pregnancy in two studies42,48 or prior to 28 weeks of pregnancy in one study53 and pregnancy loss encompassing miscarriage and ectopic pregnancy in one study44. The remaining 27 studies did not provide a definition. The odds of miscarriage were higher in women with PCOS compared with women without PCOS (OR: 1.49; 95% CI: 1.20, 1.85). Sensitivity analysis showed that after exclusion of studies in which women were taking metformin after conception20 or conceived after bariatric surgery21, miscarriage remained higher in women with PCOS (1.53; 1.23, 1.92). Cumulative meta-analysis suggested that the odds of miscarriage in women with and without PCOS did not substantially change over time. The higher odds of miscarriage were retained in post-ART pregnancies22,23,24,25,26,27,28,29,31,34,35,36,37,38,39,40,42,43,44,45,46,47,48,49,50,51,52,54, prospective22,23,24,27,31,34,35,37,38,39,43,45,62,63,65,66,78,89,94 and high-quality studies25,36,48,63,65,66. In seven studies, women with and without PCOS were matched for age22,36,48,63,66,77,78, and in six, for both age and BMI36,48,63,66,77,78; PCOS was associated with increased odds of miscarriage in age-matched or age- and BMI-matched studies.
Gestational diabetes
Fifty-eight studies reported GDM in 10011 women with PCOS and 57041 women without PCOS14,15,16,17,18,19,20,21,25,32,33,41,42,44,49,53,59,62,63,65,66,68,69,70,71,72,73,74,75,76,77,78,80,81,82,83,88,92,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113. Three studies were excluded from the meta-analysis because of overlapping participants15,62,109. Inconsistent definitions were provided for GDM across the studies. The odds of GDM were higher in women with PCOS compared with women without PCOS (OR: 2.41; 95% CI: 1.95, 2.99). Sensitivity analysis showed that after exclusion of studies in which women were taking metformin after conception16,17,18,19,20 or conceived after bariatric surgery21, the odds of GDM remained higher in PCOS (2.46; 1.99, 3.04). Cumulative meta-analysis suggested that the odds of GDM in women with and without PCOS did not substantially change over time. The higher odds of GDM were retained in post-ART pregnancies25,32,33,41,42,44,49,53,95, prospective63,65,66,70,73,75,76,78,83,88,94,100,102,104,105,107,110 and high-quality studies25,59,63,65,66,68,69. In 14 studies, women with and without PCOS were matched for age32,59,63,66,68,70,71,72,73,74,75,76,77,78 and in seven for both age and BMI63,66,68,72,73,77,78; PCOS was associated with increased odds of GDM in age-matched or age- and BMI-matched studies.
Gestational hypertension
Forty-one studies reported gestational hypertension in 5978 women with PCOS and 32230 women without PCOS17,18,20,22,32,41,49,56,57,58,59,60,61,62,63,65,66,68,69,71,74,76,78,82,83,88,92,94,95,97,98,102,103,105,107,109,111,112,113,114,115. Two studies were excluded from the meta-analysis because of overlapping participants62,109. Gestational hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg in five studies65,69,74,88,104, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg after 20 weeks of pregnancy in nine studies18,59,66,76,78,81,83,103,113, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg at two occasions more than a day apart in one study107, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg at two occasions more than six hours apart after 20 weeks of pregnancy in one study114, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg at two occasions more than six hours apart after 20 weeks of pregnancy and normalized by four to six weeks postpartum in one study60, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg at two occasions after 20 weeks of pregnancy or systolic blood pressure ≥ 150 mmHg or diastolic blood pressure ≥ 100 mmHg during labor in two studies32,71, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg without proteinuria after 20 weeks of pregnancy in two studies18,41, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg with or without proteinuria after 20 weeks of pregnancy in two studies92,95, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg with or without proteinuria68 in the third trimester in two studies97, and diastolic blood pressure > 90 mmHg at two occasions over pregnancy in one study98. The remaining 22 studies did not provide a definition. The odds of gestational hypertension were higher in women with PCOS compared with women without PCOS (OR: 2.20; 95% CI: 1.81, 2.69). Sensitivity analysis showed that after exclusion of studies in which women were taking metformin after conception17,18,20, gestational hypertension remained higher in PCOS (2.22; 1.83, 2.70). Cumulative meta-analysis suggested that the odds of gestational hypertension in women with and without PCOS did not substantially change over time; however, two studies published before 2001 contributed to heterogeneity. The higher odds of gestational hypertension were retained in post-ART pregnancies32,41,49,95, prospective56,58,60,61,63,65,66,76,78,83,88,94,102,105,107 and high-quality studies56,59,60,61,63,65,66,68,69. Three studies reported gestational hypertension in pregnancies with GDM56,57,58; the odds of gestational hypertension were higher in women with PCOS (2.57; 1.71, 3.88). In 12 studies, women with and without PCOS were matched for age32,56,59,60,61,63,66,68,71,74,76,78 and in seven for both age and BMI56,60,61,63,66,68,78; PCOS was associated with increased odds of gestational hypertension in age-matched or age- and BMI-matched studies.
Pre-eclampsia
Thirty-seven studies reported pre-eclampsia in 4999 women with PCOS and 24660 women without PCOS14,15,16,20,21,32,41,55,56,57,58,60,61,62,63,66,69,71,74,77,78,81,82,83,88,94,97,98,102,103,105,107,108,109,112,113,116. Three studies were excluded from the meta-analysis because of overlapping participants15,62,109. Pre-eclampsia was defined as gestational hypertension along with proteinuria in three studies66,78,116, gestational hypertension along with proteinuria >2+ by urine stick in two studies16,98, gestational hypertension along with proteinuria >300 mg in 24 h in thirteen studies14,32,55,58,60,69,74,75,77,83,88,103,104, gestational hypertension along with proteinuria >300 mg in 24 h or ≥1+ by urine stick in one study102, gestational hypertension along with proteinuria or end-organ dysfunction in two studies41,113, gestational hypertension along with proteinuria >300 mg in 24 h or a spot urine protein/creatinine ratio of ≥30 mg/mmol or when the hemolysis elevated liver enzymes low platelets syndrome was present in one study107, gestational hypertension along with proteinuria 100 mg/dL or >2+ in at least two random urine specimens collected six or more hours apart or >300 mg in 24 h and/or pathologic edema in three studies71,81,115. The remaining 22 studies did not provide a definition. The odds of pre-eclampsia were higher in women with PCOS compared with women without PCOS (OR: 2.30; 95% CI: 1.87, 2.82). Sensitivity analysis showed that, after exclusion of studies in which women were taking metformin after conception16,20 or conceived after bariatric surgery21, pre-eclampsia remained higher in PCOS (2.36; 1.93, 2.90). Cumulative meta-analysis suggested a diminishing magnitude in the odds of pre-eclampsia in women with and without PCOS over time.
The higher odds of pre-eclampsia were retained in post-ART pregnancies32,41 and prospective56,58,60,61,63,66,78,83,88,94,102,105,107,116 and high-quality studies56,60,61,63,66,69. Four studies reported pre-eclampsia in pregnancies with GDM55,56,57,58; the odds of pre-eclampsia were higher in women with PCOS (2.72; 1.77, 4.19). In 10 studies, women with and without PCOS were matched for age32,56,60,61,63,66,71,74,77,78 and in seven for both age and BMI56,60,61,63,66,77,78; PCOS was associated with increased odds of pre-eclampsia in age-matched or age- and BMI-matched studies.
Eclampsia
Two studies reported eclampsia in 46 women with PCOS and 246 women without PCOS32,60. One study reported no pregnancies affected by eclampsia60; therefore, only one study32 in post-ART pregnancies was used in the meta-analysis for generating the OR. The odds of eclampsia were similar in women with and without PCOS (OR: 1.16; 95% CI: 0.44, 3.08). Participants in this study were not taking metformin after conception.
Induction of labor
Eight studies reported the induction of labor in 1029 women with PCOS and 10052 women without PCOS14,21,56,82,102,104,105,107. The odds of induction of labor were similar in women with and without PCOS (OR: 1.62; 95% CI: 0.97, 2.70). Sensitivity analysis showed that after exclusion of a study in which women conceived after bariatric surgery21, the induction of labor was higher in the PCOS group (1.69; 1.01, 2.85). Cumulative meta-analysis suggested that the odds of induction of labor in women with and without PCOS did not substantially change over time; however, one study published before 2012 contributed to heterogeneity. No studies reported the induction of labor in post-ART pregnancies. The odds of induction of labor were higher in prospective studies56,102,104,105,107 but similar in a single high-quality, age- and BMI-matched study that reported outcomes in pregnancies with GDM56.
Instrumental delivery
Ten studies reported instrumental delivery in 1517 women with PCOS and 8570 women without PCOS21,56,62,66,72,98,103,105,111,116. The odds of instrumental delivery were similar in women with and without PCOS (OR: 1.18; 95% CI: 0.91, 1.53). Sensitivity analysis showed that after exclusion of a study in which women conceived after bariatric surgery21, instrumental delivery remained similar in women with and without PCOS (1.17; 0.90, 1.52). Cumulative meta-analysis suggested that the odds of instrumental delivery in women with and without PCOS did not substantially change over time. The odds of instrumental delivery were similar in prospective56,62,66,105,116 and high-quality studies56,66,88. No studies reported instrumental delivery in post-ART pregnancies. Only one study reported instrumental delivery in pregnancies with GDM56, whereby the odds were similar in women with and without PCOS (1.35; 0.22, 8.41). In four studies, women with and without PCOS were matched for both age and BMI56,66,72,88, in which PCOS was not associated with instrumental delivery.
Cesarean section
Thirty-seven studies reported cesarean section in 3590 women with PCOS and 23017 women without PCOS14,17,21,25,33,38,41,44,45,53,56,57,58,59,62,63,66,68,71,74,78,82,85,86,94,95,97,98,102,103,104,105,106,107,111,116,117. The odds of cesarean section were higher in women with PCOS compared with women without PCOS (OR: 1.23; 95% CI: 1.06, 1.43). Sensitivity analysis showed that after exclusion of studies in which women were taking metformin after conception17 or conceived after bariatric surgery21, cesarean section remained higher in PCOS (1.23; 1.06, 1.44). Convergence could not be achieved during tau2 estimation in random-effects cumulative meta-analysis. The higher odds of cesarean section were retained in prospective38,56,58,62,63,66,78,85,94,102,104,105,107,116,117 and high-quality studies25,56,59,62,63,66,68 but were not retained for post-ART pregnancies25,33,38,40,41,44,53. Three studies reported cesarean section in pregnancies with GDM56,57,58, the odds of which were higher in women with PCOS (1.69; 1.19, 2.39). In nine studies, women with and without PCOS were matched for age56,59,62,63,66,68,71,74,78 and in six for both age and BMI56,62,63,66,68,78; PCOS was associated with increased odds of cesarean section in age-matched or age- and BMI-matched studies.
Perinatal depression
One study reported perinatal depression in 3590 women with PCOS and 23017 women without PCOS118, with similar odds between groups (OR: 1.58; 95% CI: 0.87, 2.88). Participants in this study were not taking metformin after conception.
Meta-regression
Significant heterogeneity (I2 > 50%) was observed for studies reporting miscarriage, GDM, gestational hypertension, gestational weight gain, induction of labor and cesarean section. There was an insufficient number of studies (<10) for the induction of labor to perform meta‐regression.
Where reported (Table 2), age was lower in women with PCOS for miscarriage and GDM. However, BMI was higher in women with PCOS for miscarriage, GDM, gestational hypertension, gestational weight gain and cesarean section. In univariate meta‐regression, variation in age was associated with increased odds of miscarriage, with a one-year increase in age being associated with 32% higher odds of miscarriage. Age reduced the tau2 value for miscarriage from 0.31 to 0.22, indicating that 29% of between-study variance may be attributable to age. However, variation in BMI across studies was not associated with increased odds of any of the outcomes in the meta‐regression.
Discussion
In this comprehensive systematic review, meta-analysis, and meta‐regression of 104 published articles in 17384 women with and 89306 women without PCOS, women with PCOS were younger, had higher BMI around conception and had higher gestational weight gain. They were more likely to experience pregnancy complications, including miscarriage, GDM, hypertension, pre-eclampsia, induction of labor, and cesarean section. Induction of labor became higher in PCOS in the sensitivity analysis, excluding women who conceived after bariatric surgery and in prospective studies. In sensitivity meta-analyses, PCOS remained associated with higher miscarriage, GDM, hypertension and pre-eclampsia in post-ART pregnancies, prospective and high-quality studies and age-matched and age- and BMI-matched studies. Cesarean section remained associated with PCOS in prospective and high-quality studies and age-matched and age- and BMI-matched studies, but it was not associated with PCOS in post-ART pregnancies.
Overweight and obesity affect approximately 70% of women in the United States and are increasing worldwide119. There is also a global increase in gestational weight gain above recommendations120, which is a key independent contributor to obesity in the female population. Suboptimal weight around conception and insufficient or excessive gestational weight gain are associated with adverse maternal and offspring outcomes121. Aging is associated with a higher weight and worsened pregnancy outcomes122. We found that women with PCOS conceive at a higher BMI compared with women without PCOS and have higher gestational weight gain. These were assessed only and for the first time in our previous systematic review and meta-analysis8 and have been reaffirmed in these updated analyses. Mean differences in gestational weight gain did not have substantial variation over time. Women with PCOS were also younger. We were underpowered for the majority of the sensitivity analyses by age-matched or age- and BMI-matched studies. Our findings suggest that the higher gestational weight gain in PCOS is likely related to factors other than PCOS status, such as age and BMI. However, the higher odds of miscarriage, GDM, gestational hypertension, pre-eclampsia, and cesarean section are probably independent of age and BMI. The 2023 International Evidence‐based PCOS Guideline2 recommends early lifestyle intervention in women with PCOS to manage weight and improve fertility and pregnancy outcomes. However, the association of PCOS with increased odds of miscarriage, GDM, gestational hypertension, pre-eclampsia and cesarean section is probably independent of age and/or BMI.
Miscarriage affects 15.3% of confirmed pregnancies worldwide123. Here, we found that women with PCOS have 49-53% higher odds of miscarriage, and these increased odds were independent of time and ART and were retained in both prospective and high-quality studies. Age was a risk factor for increasing the odds of miscarriage independent of PCOS in univariate meta‐regression. Our findings of increased odds of miscarriage in PCOS and the association with age are consistent with prior literature8 and are not reported in other systematic reviews9,10,11,12. Here, we report for the first time that higher odds of miscarriage were observed in a younger population of women with PCOS. While ovulation rates improve in women with PCOS as they age, successful pregnancy and live births decrease with age124. This highlights that the odds of miscarriage would probably be even higher in PCOS if age was similar between the two groups. The magnitude for higher odds of miscarriage in PCOS decreased in post-ART pregnancies, but inconsistent with our original meta-analysis8, it remained higher in PCOS, with significant heterogeneity. However, the recent findings reported herein are based on a larger number of included studies and more accurate PCOS diagnosis and thus are likely more reliable.
Gestational diabetes affects up to 30% of pregnancies, depending on diagnostic criteria and ethnicity125. It is associated with increased rates of cesarean section, preterm labor, macrosomia, and large-for-gestational age offspring126 and with increased maternal risk for cardiovascular and cerebrovascular diseases later in life127. We found that PCOS is associated with 2-3-fold higher odds of GDM, and the increased odds were independent of time and ART and were retained in both prospective and high-quality studies. Where information was provided, women with PCOS were younger and had a higher BMI than women without PCOS. In those with PCOS, neither age nor BMI was associated with increased odds of GDM in the adjusted analysis, highlighting that the higher odds of GDM in PCOS are likely due to factors other than increased BMI. The magnitude for higher odds of GDM in PCOS decreased in post-ART pregnancies, but inconsistent with our prior meta-analysis8, it remained higher in PCOS. The attenuated odds are probably due to BMI restrictions for ART and poorer treatment outcomes in individuals with higher BMI128. Compared to the previous review, however, the validity of findings in this review is increased by the larger number of included studies and more accurate PCOS diagnosis. The 2023 International Evidence‐based PCOS Guideline recommends that women with PCOS are offered an oral glucose tolerance test (OGTT) for early diagnosis and intervention, ideally at preconception; if not performed at preconception, at the first antenatal visit and then repeated at 24-28 weeks of pregnancy.
Hypertensive disorders of pregnancy affect 8.5-16.7% of pregnancies, depending on ethnicity129. Gestational hypertension and pre-eclampsia are leading causes of pregnancy-related maternal mortality and are associated with 2-3-fold increases in long-term mortality from maternal cardiovascular disease130. We found that PCOS is associated with 2-3-fold higher odds of hypertensive disorders of pregnancy, independent of ART, and this was retained in both prospective and high-quality studies. Over time, the odds of pre-eclampsia diminished but remained higher in PCOS. This could be due to later studies including younger women with PCOS131 who are less likely to have chronic hypertension132. Where information was provided, women with PCOS were of similar age as women without PCOS and had higher BMI, with neither being associated with increased odds of gestational hypertension on meta‐regression. This highlights that the higher odds of hypertensive disorders of pregnancy in PCOS were due to factors other than increased BMI. We confirm the findings of our previous meta-analysis8, which showed that the higher odds of hypertensive disorders of pregnancy are likely independent of, but worsened by, GDM; however, we were underpowered for this sensitivity analysis. The higher odds of hypertensive disorders of pregnancy in PCOS were retained in post-ART pregnancies. This is inconsistent with the findings of our previous meta-analysis8, although the results are likely more reliable in the present review given the larger number of studies included here. Based on these findings, the 2023 International Evidence‐based PCOS Guideline2 has recommended monitoring of blood pressure in women with PCOS, ideally commencing in the preconception period.
Although spontaneous vaginal delivery is the most common type of delivery, the rates of induction of labor, instrumental delivery and cesarean section are increasing133. Induced and/or operative deliveries are indicated in high-risk pregnancies for mothers or offspring. The underlying conditions and the interventions may both increase the risk of hemorrhage, infection, and rehospitalization134. We found that women with PCOS have higher odds of cesarean section, which were retained in both prospective and high-quality studies. However, the odds were similar in post-ART pregnancies in women with and without PCOS. For the induction of labor and instrumental delivery, the odds were similar in women with and without PCOS, which may have been masked by the increased odds of cesarean section in women with PCOS. Our findings are consistent with the findings of a prior systematic review8. While the number of included studies was small, the higher odds of cesarean section appear to be independent of but worsened by GDM, which is consistent with our previous meta-analysis8.
The strengths of this systematic review and meta-analysis are the inclusion of the largest number of observational studies and participants across five continents to date. We excluded less reliable diagnostic methods such as self-reported PCOS status and ICD codes, as these can introduce inaccuracies due to recall bias or misinterpretation of the diagnosis and the inherent variability in diagnostic criteria across medical organizations135,136. Coupled with the heterogeneous nature of PCOS presentations, these less reliable methods can increase the potential for misdiagnosis137,138, thus capturing those with more severe symptoms and/or those not meeting the diagnostic criteria and distorting the findings on pregnancy outcomes. For the first time, we reported the mean age and BMI corresponding to each outcome of interest and performed cumulative analyses to assess the potential impact of time on the outcomes. We performed sensitivity analyses to assess the potential impact of confounders, and we also performed meta‐regression to assess the impact of age and BMI where substantial heterogeneity was observed, a pioneering approach from our previous systematic review. The certainty of evidence was assessed for individual outcomes using the GRADE approach. A limitation of this review is the inclusion of studies that were published in English, which contributes to potential language bias since English studies are more likely to demonstrate positive findings. Other limitations include the unclear or inconsistent definitions for some of the outcomes; small number of studies for sensitivity analyses by age-matched, BMI-matched and high-quality studies; and the insufficient number of studies for meta-analyses on eclampsia and perinatal depression. Furthermore, 64.1% of the included studies were of moderate to high risk of bias mainly due to high confounding bias (57.7%) followed by high selection bias (31.7%); however, we conducted sensitivity analyses of high-quality studies that confirmed the overall findings for the majority of outcomes. As this was a meta-analysis of aggregate-level data, we could only explore confounding factors in relation to age, BMI, or post-ART populations; hence, other potentially important confounders could not be accounted for.
In this systematic review and meta-analysis, we found that women with PCOS are more likely to experience pregnancy complications, which are independent of but likely exacerbated by increased age and BMI and by assisted conception. The increased odds for pregnancy complications were impacted by the quality of the studies. Women with PCOS are more likely to have pregnancy-related obesity, which could be both a risk factor for and a consequence of metabolic disorders in pregnancy. This systematic review and meta-analysis of 106690 pregnancies in women with and without PCOS directly informs the 2023 International Evidence‐based PCOS Guideline recommendation to consider PCOS as a risk factor for pregnancy complications in preconception and in antenatal care guidelines to enable timely screening, proper monitoring, and early intervention. Additional risk factors, such as age, BMI and ART, should be considered in women with PCOS. Individual patient data meta-analyses with sufficient statistical power are warranted to further investigate the impact of PCOS features and to clarify important confounders and sources of heterogeneity in the relationship between PCOS and pregnancy outcomes.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis is an update of previously published systematic reviews8,139 and was conducted in accordance with the Meta‐Analyses and Systematic Reviews of Observational Studies (MOOSE) guidelines140. Given that the majority of studies on pregnancy outcomes in prospectively identified PCOS come from selected populations with a high-risk profile141, we did not prioritize the distinction between retrospective and prospective study designs; rather, our emphasis was on the overall quality of the studies. The protocol for the original review was prospectively registered in PROSPERO (CRD 42017067147). The methods and results of the previous search with the inclusion of publications up to the 4th of April 2017 have been previously published8,139. The search was limited to English language studies and updated (MBK) from 2017 to 13th of July 2022 through Medline, Medline in‐process, and other non‐indexed citations, EMBASE, and all EBM reviews, including Cochrane Database of Systematic Reviews, Cochrane Clinical Answers, Cochrane Central Register of Controlled Trials, American College of Physicians Journal Club, Cochrane Methodology Register, Health Technology Assessments, The Database of Abstracts of Reviews of Effectiveness and the National Health Service Economic Evaluation Database. Bibliographies of recent and relevant systematic reviews and meta-analyses were searched to identify additional studies. As was done previously, search terms included a broader number of outcomes, distributed to two systematic reviews and meta-analyses8,139.
Eligible studies included studies reporting observational data on gestational weight gain, miscarriage, GDM, gestational hypertension, pre-eclampsia, eclampsia, induction of labor, instrumental delivery, cesarean section, and perinatal depression in women with and without PCOS. In a protocol amendment, we only included studies in which the criteria used for PCOS diagnosis fulfilled the Rotterdam criteria136. Pregnancy outcomes were accepted according to the primary studies’ definitions.
Studies published in languages other than English; case reports, case series, editorials, scoping and narrative reviews; studies using self-reported or International Classification of Diseases (ICD) for PCOS diagnosis; and studies not reporting the outcomes of interest in the two groups of PCOS versus non-PCOS were excluded.
Two reviewers (MBK, SH, or SS) independently screened studies by titles and abstracts and reviewed full texts for eligibility. Discrepancies were discussed and resolved through consensus or arbitration between reviewers. Studies identified through the new search were combined with those published before April 2017. Studies not meeting the new criteria needed for PCOS diagnosis were excluded (i.e., studies captured in the 2017 reviews8,139 that used self-report or ICD for PCOS diagnosis were excluded from the current review).
Data analysis
Data extraction and quality appraisal were independently performed by the same reviewers. Using an a priori researcher‐developed data extraction form, data were extracted from each study. These included the author, year of publication, study design, study location, participant characteristics, and frequency/mean and standard deviation of outcomes per group. Participant characteristics included age, BMI, mode of conception, existing medical conditions, and medications used during pregnancy.
When participants were overlapping between multiple publications for the same outcome, data from the study with the largest sample size were included in the meta-analysis.
the risk of bias was independently assessed by two reviewers (MBK, SH, or SS) using the Newcastle‐Ottawa Scale (NOS) for non‐randomized studies for selection, comparability, and outcome ascertainment142. Studies were considered high-quality if they scored at least three stars in selection, one star in comparability, and two stars in outcome ascertainment. Studies with two stars in selection, a minimum of one star in comparability, and two stars in outcome ascertainment were considered fair quality. Studies meeting neither of these two thresholds were considered low quality.
We performed random-effects meta-analyses to generate pooled effect estimates, reported as the mean differences (MDs) or odds ratios (ORs) with 95% confidence intervals (CIs), for the association between PCOS status and pregnancy outcomes. Between-study heterogeneity was assessed using the I² statistic; I² above 50% was deemed substantial heterogeneity143. Sensitivity analyses were performed, excluding studies in which participants were taking metformin after conception or conceived after bariatric surgery. Cumulative random-effects meta‐analyses based on Hedge’s adjusted g and its 95% confidence intervals (95% CI)144 were performed to explore the effect of time (publication year) on the association of PCOS with each outcome of interest. Publication bias was assessed using funnel plots. We assessed the certainty of evidence for each outcome according to the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system145 using Gradepro software146.
Sensitivity analyses of the outcomes by conception with ART (in vitro fertilization, in vitro maturation intracytoplasmic sperm injection, zygote intrafallopian transfer, and gamete intro-fallopian transfer) and prospective and high-quality studies as well as by age- and BMI-matched designs were also performed after exclusion of studies in which women were taking metformin after conception or conceived after bariatric surgery.
Restricted maximum likelihood (REML)‐based random effects meta‐regression was performed to explore the effects of variations in age and BMI on each outcome of interest if there were equal to or greater than 10 studies per coefficient. Effect sizes of age and BMI for individual studies were used in meta‐regression analyses. The percentage of between-study variance explained by the model (tau2) was estimated using the Knapp–Hartung modification. Normal distributions for mean values were checked using skewness‐kurtosis tests. As age and BMI were not both significant (p < 0.01) for any of the outcomes on univariate meta‐regression analyses, multivariable meta‐regression could not be performed. Statistical significance was defined as two-sided P < 0.05. All statistical analyses were performed using Stata version 17 (StataCorp, 14 College Station, Texas, USA).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data that support the findings of this study have been deposited in https://data.mendeley.com/datasets/npy96r94p2/1 (https://doi.org/10.17632/npy96r94p2.1). Source data are provided with this paper.
References
Bozdag, G., Mumusoglu, S., Zengin, D., Karabulut, E. & Yildiz, B. O. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 31, 2841–2855 (2016).
Teede, H. J. et al. Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 108, 2447–2469 (2023).
Lim, S. S., Davies, M. J., Norman, R. J. & Moran, L. J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. Update 18, 618–637 (2012).
Lim, S. S., Norman, R. J., Davies, M. J. & Moran, L. J. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes. Rev. 14, 95–109 (2013).
Yin, X., Ji, Y., Chan, C. L. W. & Chan, C. H. Y. The mental health of women with polycystic ovary syndrome: a systematic review and meta-analysis. Arch. Women’s Ment. Health 24, 11–27 (2021).
Balen, A. H. et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 22, 687–708 (2016).
Piltonen, T. T. et al. Awareness of polycystic ovary syndrome among obstetrician-gynecologists and endocrinologists in Northern Europe. PLoS ONE 14, e0226074 (2019).
Bahri Khomami, M. et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity—A systematic review, meta‐analysis, and meta‐regression. Obes. Rev. 20, 659–674 (2019).
Boomsma, C. M. et al. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Update 12, 673–683 (2006).
Kjerulff, L. E., Sanchez-Ramos, L. & Duffy, D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am. J. Obstet. Gynecol. 204, 558.e551–556 (2011).
Qin, J. Z. et al. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod. Biol. Endocrinol. 11, 56 (2013).
Yu, H. F., Chen, H. S., Rao, D. P. & Gong, J. Association between polycystic ovary syndrome and the risk of pregnancy complications: A PRISMA-compliant systematic review and meta-analysis. Med. (Baltim.) 95, e4863 (2016).
Orwin, R. G. Evaluating coding decisions. In The Handbook of Research Synthesis (eds Cooper, H. & Hedges, L. V.) pp. 139–162 (Russell Sage Foundation, 1994).
Jonsdottir, F. et al. Obstetrical complications in dichorionic twin pregnancies in women with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 96, 1453–1459 (2017).
Glueck, C. J. et al. Metformin, pre-eclampsia, and pregnancy outcomes in women with polycystic ovary syndrome. Diabet. Med 21, 829–836 (2004).
Glueck, C. J. et al. Height, weight, and motor-social development during the first 18 months of life in 126 infants born to 109 mothers with polycystic ovary syndrome who conceived on and continued metformin through pregnancy. Hum. Reprod. 19, 1323–1330 (2004).
Kovo, M. et al. Neonatal outcome in polycystic ovarian syndrome patients treated with metformin during pregnancy. J. Matern. Fetal Neonatal. Med. 19, 415–419 (2006).
Sir-Petermann, T. et al. Serum adiponectin and lipid concentrations in pregnant women with polycystic ovary syndrome. Hum. Reprod. 22, 1830–1836 (2007).
Bolton, S., Cleary, B., Walsh, J., Dempsey, E. & Turner, M. J. Continuation of metformin in the first trimester of women with polycystic ovarian syndrome is not associated with increased perinatal morbidity. Eur. J. Pediatr. 168, 203–206 (2009).
De Leo, V., Musacchio, M. C., Piomboni, P., Di Sabatino, A. & Morgante, G. The administration of metformin during pregnancy reduces polycystic ovary syndrome related gestational complications. Eur. J. Obstet. Gynecol. Reprod. Biol. 157, 63–66 (2011).
Benito, E. et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J. Clin. Endocrinol. Metab. 105, 01 (2020).
Urman, B. et al. The outcome of in vitro fertilization and embryo transfer in women with polycystic ovary syndrome failing to conceive after ovulation induction with exogenous gonadotropins. Fertil. Steril. 57, 1269–1273 (1992).
Homburg, R. et al. In vitro fertilization and embryo transfer for the treatment of infertility associated with polycystic ovary syndrome. Fertil. Steril. 60, 858–863 (1993).
Wang, J. X., Davies, M. J. & Norman, R. J. Polycystic ovarian syndrome and the risk of spontaneous abortion following assisted reproductive technology treatment. Hum. Reprod. 16, 2606–2609 (2001).
Dokras, A. et al. Obstetric outcomes after in vitro fertilization in obese and morbidly obese women. Obstet. Gynecol. 108, 61–69 (2006).
Beydoun, H. A. et al. Impact of polycystic ovary syndrome on selected indicators of in vitro fertilization and intracytoplasmic sperm injection treatment success. J. Women’s Health (Larchmt.) 18, 717–723 (2009).
Nejad, E. S. et al. Comparison of in vitro fertilisation success in patients with polycystic ovary syndrome and tubal factor. Gynecol. Endocrinol. 27, 117–120 (2011).
Huang, K., Dong, X., Zhang, H. & Liao, X. Effect of overweight/obesity on IVF-ET outcomes in chinese patients with polycystic ovary syndrome. Int. J. Clin. Exp. Med. 7, 5872–5876 (2014).
Li, H. W. et al. Cumulative live-birth rate in women with polycystic ovary syndrome or isolated polycystic ovaries undergoing in-vitro fertilisation treatment. J. Assist Reprod. Genet. 31, 205–211 (2014).
Liu, L. et al. A comparison of the miscarriage rate between women with and without polycystic ovarian syndrome undergoing IVF treatment. Eur. J. Obstet. Gynecol. Reprod. Biol. 176, 178–182 (2014).
Zhang, C. M. et al. Metabolic heterogeneity of follicular amino acids in polycystic ovary syndrome is affected by obesity and related to pregnancy outcome. BMC Pregnancy Childbirth 14, 11 (2014).
Wan, H. L., Hui, P. W., Li, H. W. & Ng, E. H. Obstetric outcomes in women with polycystic ovary syndrome and isolated polycystic ovaries undergoing in vitro fertilization: a retrospective cohort analysis. J. Matern. Fetal Neonatal. Med. 28, 475–478 (2015).
Sterling, L. et al. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil. Steril. 105, 791–797.e792 (2016).
Wang, F., Dai, W., Yang, X. H., Guo, Y. H. & Sun, Y. P. Analyses of optimal body mass index for infertile patients with either polycystic or non-polycystic ovary syndrome during assisted reproductive treatment in China. Science 6, 34538 (2016).
Chen, Y. et al. Predicting the outcome of different protocols of in vitro fertilization with anti-Muullerian hormone levels in patients with polycystic ovary syndrome. J. Int. Med. Res. 45, 1138–1147 (2017).
Luo, L. et al. Early miscarriage rate in lean polycystic ovary syndrome women after euploid embryo transfer—a matched-pair study. Reprod. Biomed. Online 35, 576–582 (2017).
Lai, Q. et al. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front. Med. 12, 518–524 (2018).
Chen, M. et al. Systematic oxidative stress is not associated with live birth rate in young non-obese patients with polycystic ovarian syndrome undergoing assisted reproduction cycles: a prospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 253, 154–161 (2020).
Elshewy, N. et al. Association between mild stimulated IVF/M cycle and early embryo arrest in sub fertile women with/without PCOS. Reprod. Biol. Endocrinol. 18, 71 (2020).
Liu, L. et al. Effect of pregravid obesity on perinatal outcomes in singleton pregnancies following in vitro fertilization and the weight-loss goals to reduce the risks of poor pregnancy outcomes: a retrospective cohort study. PLoS ONE [Electron. Resour.] 15, e0227766 (2020).
Abdulkhalikova, D. et al. Perinatal outcome of in vitro fertilization pregnancies in women with polycystic ovary syndrome by pregravid BMI. J. Perinat. Med. 49, 514–519 (2021).
Cai, H. et al. Early and late pregnancy loss in women with polycystic ovary syndrome undergoing IVF/ICSI treatment: a retrospective cohort analysis of 21 820 pregnancies. Bjog 128, 1160–1169 (2021).
Gongadashetti, K., Gupta, P., Dada, R. & Malhotra, N. Follicular fluid oxidative stress biomarkers and art outcomes in PCOS women undergoing in vitro fertilization: A cross-sectional study. Int. J. Reprod. BioMed. 19, 449–456 (2021).
Hu, S., Xu, B., Long, R. & Jin, L. The effect of polycystic ovary syndrome without hyperandrogenism on pregnancy-related outcomes: a retrospective cohort study. Bjog 128, 1003–1010 (2021).
Liu, Y. et al. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J. Assist. Reprod. Genet. 38, 471–477 (2021).
Mai, Z. et al. Comparison of cumulative live birth rate between aged PCOS women and controls in IVF/ICSI cycles. Front. Endocrinol. (Lausanne) 12, 724333 (2021).
Pouya, K. et al. hCG day progesterone level has no impact on the frozen thawed embryo transfer cycle outcome. J. Gynecol. Obstet. Hum. Reprod. 50, 102120 (2021).
Wu, P. F., Li, R. Z., Zhang, W., Su, Z. Z. & Lin, Y. H. Polycystic ovary syndrome is not associated with offspring birth weight: a mendelian randomization study. Biomed. Environ. Sci. 34, 170–174 (2021).
Zhu, J. et al. A comprehensive evaluation of progestin-primed ovarian stimulation protocol in patients with or without PCOS undergoing in vitro fertilization. Reprod. Biol. 21, 100540 (2021).
Ni, Z. et al. Adverse effects of polycystic ovarian syndrome on pregnancy outcomes in women with frozen-thawed embryo transfer: propensity score-matched study. Front. Endocrinol. (Lausanne) 13, 878853 (2022).
Song, H., Yu, Z., Li, P., Wang, Y. & Shi, Y. HOMA-IR for predicting clinical pregnancy rate during IVF. Gynecol. Endocrinol. 38, 33–38 (2022).
Tu, M. et al. Effect of lncRNA MALAT1 on the granulosa cell proliferation and pregnancy outcome in patients with PCOS. Front. Endocrinol. (Lausanne) 13, 825431 (2022).
Wang, Q. et al. Association of polycystic ovary syndrome phenotypes with adverse pregnancy outcomes after in-vitro fertilization/intracytoplasmic sperm injection. Front. Endocrinol. (Lausanne) 13, 889029 (2022).
Yang, T. et al. Homeostatic model assessment for insulin resistance is associated with late miscarriage in non-dyslipidemic women undergoing fresh IVF/ICSI embryo transfer. Front. Endocrinol. (Lausanne) 13, 880518 (2022).
Li, G., Fan, L., Zhang, L., Zhang, W. & Huang, X. Metabolic parameters and perinatal outcomes of gestational diabetes mellitus in women with polycystic ovary syndrome.[Erratum appears in J Perinat Med. 2010 May;38(3):343]. J. Perinat. Med. 38, 141–146 (2010).
Palomba, S. et al. The risk of a persistent glucose metabolism impairment after gestational diabetes mellitus is increased in patients with polycystic ovary syndrome. Diabetes Care 35, 861–867 (2012).
Foroozanfard, F., Moosavi, S. G., Mansouri, F. & Bazarganipour, F. Obstetric and neonatal outcome in PCOS with gestational diabetes mellitus. J. Fam. Reprod. Health 8, 7–12 (2014).
Aktun, H. L., Yorgunlar, B., Acet, M., Aygun, B. K. & Karaca, N. The effects of polycystic ovary syndrome on gestational diabetes mellitus. Gynecol. Endocrinol. 32, 139–142 (2016).
Haakova, L. et al. Pregnancy outcome in women with PCOS and in controls matched by age and weight. Hum. Reprod. 18, 1438–1441 (2003).
Hu, S., Leonard, A., Seifalian, A. & Hardiman, P. Vascular dysfunction during pregnancy in women with polycystic ovary syndrome. Hum. Reprod. 22, 1532–1539 (2007).
Falbo, A. et al. Changes in androgens and insulin sensitivity indexes throughout pregnancy in women with polycystic ovary syndrome (PCOS): Relationships with adverse outcomes. J. Ovarian Res. 3 (2010).
Palomba, S. et al. Uterine blood flow in pregnant patients with polycystic ovary syndrome: relationships with clinical outcomes. Bjog 117, 711–721 (2010).
Nouh, A. A. & Shalaby, S. M. The predictive value of uterine blood flow in detecting the risk of adverse pregnancy outcome in patients with polycystic ovary syndrome. Middle East Fertil. Soc. J. 16, 284–290 (2011).
Mehrabian, F. & Kelishadi, R. Comparison of the metabolic parameters and androgen level of umbilical cord blood in newborns of mothers with polycystic ovary syndrome and controls. J. Res. Med. Sci. 17, 207–211 (2012).
Wang, Y. et al. Risks for gestational diabetes mellitus and pregnancy-induced hypertension are increased in polycystic ovary syndrome. Biomed. Res. Int. 2013, 182582 (2013).
Palomba, S. et al. Lipid profile in nonobese pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. Steroids 88, 36–43 (2014).
Palomba, S. et al. Low-grade chronic inflammation in pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. J. Clin. Endocrinol. Metab. 99, 2942–2951 (2014).
Sawada, M. et al. Pregnancy complications and glucose intolerance in women with polycystic ovary syndrome. Endocr. J. 62, 1017–1023 (2015).
Zheng, W. et al. Early pregnancy metabolic factors associated with gestational diabetes mellitus in normal-weight women with polycystic ovary syndrome: a two-phase cohort study. Diabetol. Metabolic Syndrome 11 (2019).
Levran, D. et al. Glucose tolerance in pregnant women following treatment for sterility. Int J. Fertil. 35, 157–159 (1990).
Fridstrom, M., Nisell, H., Sjoblom, P. & Hillensjo, T. Are women with polycystic ovary syndrome at an increased risk of pregnancy-induced hypertension and/or preeclampsia? Hypertens 18, 73–80 (1999).
Vollenhoven, B., Clark, S., Kovacs, G., Burger, H. & Healy, D. Prevalence of gestational diabetes mellitus in polycystic ovarian syndrome (PCOS) patients pregnant after ovulation induction with gonadotrophins. Aust. N. Z. J. Obstet. Gynaecol. 40, 54–58 (2000).
Sir-Petermann, T. et al. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum. Reprod. 17, 2573–2579 (2002).
Weerakiet, S. et al. Prevalence of gestational diabetes mellitus and pregnancy outcomes in Asian women with polycystic ovary syndrome. Gynecol. Endocrinol. 19, 134–140 (2004).
Sir-Petermann, T. et al. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum. Reprod. 20, 2122–2126 (2005).
Gupta, A., Raina, K., Kalkkar, T. & Veer, Y. Pregnancy outcome in women with the polycystic ovarian syndrome. JK Sci. 11, 82–84 (2009).
Reyes-Munoz, E. et al. The risk of gestational diabetes mellitus among Mexican women with a history of infertility and polycystic ovary syndrome. Fertil. Steril. 97, 1467–1471 (2012).
Elkholi, D. G. E. Y. & Nagy, H. M. The effects of adipocytokines on the endocrino-metabolic features and obstetric outcome in pregnant obese women with polycystic ovary syndrome. Middle East Fertil. Soc. J. 19, 293–302 (2014).
Mousa, A., Tay, C. & Teede, H. Technical Report for the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. (2023).
Lesser, K. B. & Garcia, F. A. Association between polycystic ovary syndrome and glucose intolerance during pregnancy. J. Matern. Fetal. Med. 6, 303–307 (1997).
Urman, B., Sarac, E., Dogan, L. & Gurgan, T. Pregnancy in infertile PCOD patients. Complications and outcome. J. Reprod. Med. 42, 501–505 (1997).
Turhan, N. O., Seckin, N. C., Aybar, F. & Inegol, I. Assessment of glucose tolerance and pregnancy outcome of polycystic ovary patients. Int. J. Gynaecol. Obstet. 81, 163–168 (2003).
Al-Ojaimi, E. H. Pregnancy outcomes after laparoscopic ovarian drilling in women with polycystic ovarian syndrome. Saudi Med. J. 27, 519–525 (2006).
Maliqueo, M. et al. Metabolic parameters in cord blood of newborns of women with polycystic ovary syndrome. Fertil. Steril. 92, 277–282 (2009).
Anderson, H. et al. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J. Clin. Endocrinol. Metab. 95, 2180–2186 (2010).
Wang, Y. et al. Development of 1-2 years offspring born to mothers with polycystic ovary syndrome. J. Coll. Physicians Surg. Pak. 31, 1186–1190 (2021).
Palep-Singh, M., Picton, H. M., Vrotsou, K., Maruthini, D. & Balen, A. H. South Asian women with polycystic ovary syndrome exhibit greater sensitivity to gonadotropin stimulation with reduced fertilization and ongoing pregnancy rates than their Caucasian counterparts. Eur. J. Obstet. Gynecol. Reprod. Biol. 134, 202–207 (2007).
Palomba, S. et al. Pregnancy in women with polycystic ovary syndrome: the effect of different phenotypes and features on obstetric and neonatal outcomes. Fertil. Steril. 94, 1805–1811 (2010).
Lathi, R. B. et al. The role of serum testosterone in early pregnancy outcome: a comparison in women with and without polycystic ovary syndrome. J. Obstet. Gynaecol. Can. 36, 811–816 (2014).
Wang, Q. et al. Low aneuploidy rate in early pregnancy loss abortuses from patients with polycystic ovary syndrome. Reprod. Biomed. Online 33, 85–92 (2016).
Huang, Q. et al. Relationship between a low ratio of serum estradiol to follicle number and fertility treatment outcomes: a retrospective cohort study of 516 cases. Medicine 97, e12017 (2018).
Li, Y. et al. Comparing the risk of adverse pregnancy outcomes of Chinese patients with polycystic ovary syndrome with and without antiandrogenic pretreatment. Fertil. Steril. 109, 720–727 (2018).
Butts, S. F. et al. Vitamin D deficiency is associated with poor ovarian stimulation outcome in PCOS but not unexplained infertility. J. Clin. Endocrinol. Metab. 104, 369–378 (2019).
Foroozanfard, F. et al. Comparing pregnancy, childbirth, and neonatal outcomes in women with different phenotypes of polycystic ovary syndrome and healthy women: a prospective cohort study. Gynecol. Endocrinol. 36, 61–65 (2020).
Liu, S. et al. Pregnancy outcomes of women with polycystic ovary syndrome for the first in vitro fertilization treatment: a retrospective cohort study with 7678 patients. Front. Endocrinol. (Lausanne) 11, 575337 (2020).
Wortsman, J., de Angeles, S., Futterweit, W., Singh, K. B. & Kaufmann, R. C. Gestational diabetes and neonatal macrosomia in the polycystic ovary syndrome. J. Reprod. Med. 36, 659–661 (1991).
Mikola, M., Hiilesmaa, V., Halttunen, M., Suhonen, L. & Tiitinen, A. Obstetric outcome in women with polycystic ovarian syndrome. Hum. Reprod. 16, 226–229 (2001).
Bjercke, S. et al. Impact of insulin resistance on pregnancy complications and outcome in women with polycystic ovary syndrome. Gynecol. Obstet. Invest 54, 94–98 (2002).
Dmitrovic, R., Katcher, H. I., Kunselman, A. R. & Legro, R. S. Continuous glucose monitoring during pregnancy in women with polycystic ovary syndrome. Obstet. Gynecol. 118, 878–885 (2011).
Boutzios, G. et al. Polycystic ovary syndrome offspring display increased oxidative stress markers comparable to gestational diabetes offspring. Fertil. Steril. 99, 943–950 (2013).
Ashrafi, M. et al. Gestational diabetes mellitus risk factors in women with polycystic ovary syndrome (PCOS). Eur. J. Obstet. Gynecol. Reprod. Biol. 181, 195–199 (2014).
Naver, K. V. et al. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. Bjog 121, 575–581 (2014).
Kollmann, M. et al. Maternal and neonatal outcomes in pregnant women with PCOS: comparison of different diagnostic definitions. Hum. Reprod. 30, 2396–2403 (2015).
Koster, M. P. et al. Placental characteristics in women with polycystic ovary syndrome. Hum. Reprod. 30, 2829–2837 (2015).
Mumm, H. et al. Hyperandrogenism and phenotypes of polycystic ovary syndrome are not associated with differences in obstetric outcomes. Acta Obstet. Gynecol. Scand. 94, 204–211 (2015).
Xiao, Q., Cui, Y. Y., Lu, J., Zhang, G. Z. & Zeng, F. L. Risk for gestational diabetes mellitus and adverse birth outcomes in chinese women with polycystic ovary syndrome. Int 2016, 5787104 (2016).
de Wilde, M. A. et al. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil. Steril. 108, 333–340 (2017).
Kent, J. et al. Gestational weight gain in women with polycystic ovary syndrome: a controlled study. J. Clin. Endocrinol. Metab. 103, 4315–4323 (2018).
Kollmann, M. et al. Androgen and anti-mullerian hormone concentrations at term in newborns and their mothers with and without polycystic ovary syndrome. J. Clin. Med. 8 (2019).
Feichtinger, M. et al. Maternal overweight vs. Polycystic ovary syndrome: Disentangling their impact on insulin action in pregnancy—a prospective study. J. Clin. Med. 10, 1–7 (2021).
Jiang, L. et al. Associations between sex hormone levels and autistic traits in infertile patients with polycystic ovary syndrome and their offspring. Front Endocrinol. (Lausanne) 12, 789395 (2021).
Kollmann, M. et al. Article vitamin D concentrations at term do not differ in newborns and their mothers with and without polycystic ovary syndrome. J. Clin. Med. 10, 1–9 (2021).
Liu, Q., Wang, J., Xu, Q., Kong, L. & Wang, J. A retrospective cohort study of obstetric complications and birth outcomes in women with polycystic ovarian syndrome. J. Obstet. Gynaecol. 42, 574–579 (2022).
Kashyap, S. & Claman, P. Polycystic ovary disease and the risk of pregnancy-induced hypertension. J. Reprod. Med. 45, 991–994 (2000).
Schneider, D., Gonzalez, J. R., Yamamoto, M., Yang, J. & Lo, J. C. The association of polycystic ovary syndrome and gestational hypertensive disorders in a diverse community-based cohort. J. Pregnancy 2019, 9847057 (2019).
Diamant, Y. Z., Rimon, E. & Evron, S. High incidence of preeclamptic toxemia in patients with polycystic ovarian disease. Eur. J. Obstet. Gynecol. Reprod. Biol. 14, 199–204 (1982).
Tobiasz, A. M., Duncan, J. R., Detti, L. & Mari, G. Lack of fetal insulin resistance in maternal polycystic ovary syndrome. Reprod. Sci. 27, 1253–1258 (2020).
March, W. A., Whitrow, M. J., Davies, M. J., Fernandez, R. C. & Moore, V. M. Postnatal depression in a community-based study of women with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 97, 838–844 (2018).
Fryar, C. D., Carroll, M. D. & Afful, J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats (2020).
Institute of M, National Research Council Committee to Reexamine IOMPWG. In Weight Gain During Pregnancy: Reexamining the Guidelines (eds. Rasmussen K. M. & Yaktine A. L.) (National Academies Press (US) Copyright © 2009, National Academy of Sciences, 2009).
Goldstein, R. F. et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. Jama 317, 2207–2225 (2017).
Fall, C. H. et al. Association between maternal age at childbirth and child and adult outcomes in the offspring: a prospective study in five low-income and middle-income countries (COHORTS collaboration). Lancet Glob. Health 3, e366–e377 (2015).
Quenby, S. et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 397, 1658–1667 (2021).
Rausch, M. E. et al. Predictors of pregnancy in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 94, 3458–3466 (2009).
McIntyre, H. D. et al. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 5, 47 (2019).
Ye, W. et al. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 377, e067946 (2022).
Xie, W. et al. Association of gestational diabetes mellitus with overall and type specific cardiovascular and cerebrovascular diseases: systematic review and meta-analysis. BMJ 378, e070244 (2022).
Kelley, A. S., Badon, S. E., Lanham, M. S. M., Fisseha, S. & Moravek, M. B. Body mass index restrictions in fertility treatment: a national survey of OB/GYN subspecialists. J. Assist Reprod. Genet. 36, 1117–1125 (2019).
Shah, N. S. et al. Trends in De Novo hypertensive disorders of pregnancy among asian and hispanic population subgroups in the United States, 2011 to 2019. JAMA Cardiol. 7, 742–746 (2022).
Ford, N. D. et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization—United States, 2017–2019. MMWR Morb. Mortal. Wkly Rep. 71, 585–591 (2022).
Funai, E. F. et al. Risk factors for pre-eclampsia in nulliparous and parous women: the Jerusalem Perinatal Study. Paediatr. Perinat. Epidemiol. 19, 59–68 (2005).
Ananth, C. V. et al. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension 74, 1089–1095 (2019).
Desai, N. M. & Tsukerman, A. Vaginal Delivery. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559197/# (2022).
Bruno, A. M. et al. Physician cesarean delivery rates and severe perinatal morbidity among low-risk nulliparas. J. Perinatol. 43, 34–38 (2023).
Herrett, E. et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J. Epidemiol. 44, 827–836 (2015).
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Hum. Reprod. 19, 41–47 (2004).
Azziz, R. et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 89, 2745–2749 (2004).
Diamanti-Kandarakis, E. et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J. Clin. Endocrinol. Metab. 84, 4006–4011 (1999).
Bahri Khomami, M. et al. The role of maternal obesity in infant outcomes in polycystic ovary syndrome—a systematic review, meta‐analysis, and meta‐regression. Obes. Rev. 20, 842–858 (2019).
Stroup, D. F. et al. Meta-analysis of observational studies in EpidemiologyA proposal for reporting. JAMA 283, 2008–2012 (2000).
Bahri Khomami, M. et al. Clinical management of pregnancy in women with polycystic ovary syndrome: an expert opinion. Clin. Endocrinol. 97, 227–236 (2022).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses (2014).
Deeks, J. J., Higgins, J. P. T. & Altman, D. G. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3, Ch. 10 (Higgins J. P. T. et al.) (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hedges, L. V. & Olkin I. Statistical Methods for Meta-analysis (Academic Press, 2014).
Guyatt, G. H. et al. Going from evidence to recommendations. Bmj 336, 1049–1051 (2008).
The GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations Updated October 2013. Available from guidelinedevelopment.org/handbook.
Acknowledgements
Eszter Vanky and Aya Mousa contributed equally as joint senior authors. We extend our gratitude to the colleagues who contributed to the initial systematic review published in 2019. While these collaborators were not directly involved in this update, their contributions to the foundational work have been instrumental in shaping the direction and scope of the current study. This work is supported by the Australian National Health and Medical Research Council (NHMRC) funded Centre for Research Excellence in Women’s Health in Reproductive Life (CRE-WHiRL) [APP#1171592] (HJT). The funder of the study had no role in study design, data extraction, data analysis, data interpretation, writing of the manuscript, or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
M.B.K. contributed to the concept and design and performed the systematic search and statistical analysis. M.B.K., S.S., and S.H. performed screening, data extraction, quality appraisal, and drafting of the paper. H.J.T. obtained funding and led the overarching guideline process. C.T.T. and A.M. were evidence synthesis leads for the guideline including this study. E.V. was the clinical expert lead in the guideline committee. M.B.K., S.S., S.H., C.H.L., T.T.P., D.R., C.T.T., H.J.T., E.V., and A.M. contributed to the concept and design and provided substantial contributions to the drafting of the work, including critical revision for important intellectual content. M.B.K., S.S., S.H., C.H.L., T.T.P., D.R., C.T.T., H.J.T., E.V., and A.M. had full access to the data, were responsible for study integrity and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
T.P. is supported by Novo Nordisk and Sigrid Jusélius Foundation. EV is supported by Novo Nordisk and Merck as a lecturer and advisor for clinical studies. H.J.T. and A.M. are supported by NHMRC fellowships [APP#2009326 and APP#1161871, respectively]. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Nicolás Crisosto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bahri Khomami, M., Shorakae, S., Hashemi, S. et al. Systematic review and meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Nat Commun 15, 5591 (2024). https://doi.org/10.1038/s41467-024-49749-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-49749-1
- Springer Nature Limited