Abstract
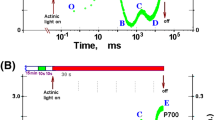
Cucumber leaf discs were illuminated at room-temperature with far-red light to photo-oxidise P700, the chlorophyll dimer in Photosystem (PS) I. The post-illumination kinetics of P700+ re-reduction were studied in the presence of inhibitors or cofactors of photosynthetic electron transport. The re-reduction kinetics of P700+ were well fitted as the sum of three exponentials, each with its amplitude and rate coefficient, and an initial flux (at the instant of turning off far-red light) given as the product of the two. Each initial flux is assumed equal to a steady state flux during far-red illumination. The fast phase of re-reduction, with rate coefficient k 1∼ 10 s−1, was completely abolished by a saturating concentration of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU); it is attributed to electron flow to P700+ from PS II, which was stimulated to some extent by far-red light. The intermediate phase, with rate coefficient k 1∼ 1 s−1, was only partly diminished by methyl viologen (MV) which diverts electron flow to oxygen. The intermediate phase is attributed to electron donation from reduced ferredoxin to the intersystem pool; reduced ferredoxin could be formed: (1) directly by electron donation on the acceptor of PS I; and/or (2) indirectly by stromal reductants, in line with only a partial inhibition of the intermediate phase by MV. Duroquinol enhanced the intermediate phase in the presence of DCMU, presumably through its interaction with thylakoid membrane components leading to the partial reduction of plastoquinone. The slow phase of P700+ re-reduction, with rate coefficient k 1∼ 0.1 s−1, was unaffected by DCMU and only slightly affected by MV; it could be associated with electron donation to either: (1) the intersystem chain by stromal reductants catalysed by NAD(P)H dehydrogenase slowly; or (2) plastocyanin/P700+ by ascorbate diffusing across the thylakoid membrane to the lumen. It is concluded that a post-illumination analysis of the fluxes to P700+ can be used to probe the pathways of electron flow to PS I in steady state illumination.
Similar content being viewed by others
References
Albertsson P-A (1995) The structure and function of the chloroplast photosynthetic membrane — a model for the domain organisation. Photosynth Res 46: 141–149
Asada K, Heber U and Schreiber U (1992) Pool size of electrons that can be donated to P700+, as determined in intact leaves: donation to P700+ from stromal components via the intersystem chain. Plant Cell Physiol 33: 927–932
Asada K, Heber U and Schreiber U (1993) Electron flow to the intersystem chain from stromal components and cyclic electron flow in maize chloroplasts, as detected in intact leaves by monitoring redox change of P700 and chlorophyll fluorescence. Plant Cell Physiol 34: 39–50
Bendall DS and Manasse RS (1995) Cyclic photophosphorylation and electron transport. Biochim Biophys Acta 1229: 23–38
Bukhov NG, Wiese C, Neimanis S and Heber U (1999) Heat sensitivity of chloroplasts and leaves: leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth Res 59: 81–93
Bukhov NG, Carpentier R and Samson G (2001a) Heterogeneity of Photosystem I reaction centers in barley leaves as related to the donation from stromal reductants. Photosynth Res 70: 273–279
Bukhov NG, Samson G and Carpentier R (2001b) Nonphotochemical reduction of the intersystem electron transport chain of chloroplasts following heat stress. The pool size of stromal reductants. Photochem Photobiol 74: 438–443
Bukhov NG, Egorova E and Carpentier R (2002) Electron flow to Photosystem I from stromal reductants in vivo: the size of the pool of stromal reductants controls the rate of electron donation to both rapidly and slowly reducing Photosystem I units. Planta 215: 812–820
Chow WS and Hope AB (1999) The electrochromic signal, redox reactions in the cytochrome bf complex and photosystem functionality in photoinhibited tobacco leaf segments. Aust J Plant Physiol 25: 775–784
Cleland RE and Bendall DS (1992) Photosystem I cyclic electron transport: measurement of ferredoxin-plastoquinone reductase activity. Photosynth Res 34: 409–418
Corneille S, Cournac L, Guedeney G, Havaux M and Peltier G (1998) Reduction of the plastoquinone pool by exogenous NADH and NADPH in higher plant chloroplasts. Characterization of a NAD(P)H-plastoquinone oxidoreductase activity. Biochim Biophys Acta 1363: 59–69
Endo T, Mi H, Shikanai T and Asada K (1997) Donation of electrons to plastoquinone by NAD(P)H dehydrogenase and by ferredoxin-quinone reductase in spinach chloroplasts. Plant Cell Physiol 38: 1272–1277
Genty B and Harbinson J (1996) Regulation of light utilization for photosynthetic electron transport. In: Baker NR (ed) Photosynthesis and the Environment, pp 67–99. Kluwer Academic Publishers, Dordrecht, The Netherlands
Groom QJ, Kramer DM, Crofts AR and Ort DR (1993) The non-photochemical reduction of plastoquinone in leaves. Photosynth Res 36: 205–215
Haehnel W and Trebst A (1982) Localization of electron transport inhibition in plastoquinone reactions. J Bioenerg Biomemb 14: 181–190
Havaux M (1996) Short-term responses of Photosystem I to heat stress. Induction of a PS II-independent electron transport through PS I fed by stromal components. Photosynth Res 47: 85–97
Heldt HW and Flügge UI (1987) Subcellular transport of metabolites in plants. In: Stumpf PK and Conn EE (eds) The Biochemistry of Plants, Vol 12, pp 49–85. Academic Press, New York
Hope AB, Liggins J and Matthews DB (1989) The kinetics of reactions in and near the cytochrome b/f complex of chloroplasts: II Cytochrome b-563 reduction. Aust J Plant Physiol 16: 353–364
Jin M-X, Yao Z-J and Mi H (2001) Multi-phasic kinetics of P700+ dark re-reduction in Nicotiana tabacum. Photosynthetica 39: 419–425
Kim S-J, Lee C-H, Hope AB and Chow WS (2001) Inhibition of Photosystems I and II and enhanced back flow of Photosystem I electrons in cucumber leaf discs chilled in the light. Plant Cell Physiol 42: 842–848
Klughammer C and Schreiber U (1991) Analysis of light-induced absorbance changes in the near-infrared spectral region. I. Characterization of various components in isolated chloroplasts. Z Naturforsch 46c: 233–244
Klughammer C and Schreiber U (1998) Measuring P700 absorbance changes in the near infrared spectral region with a dual wavelength pulse modulation system. In: Garab G (ed) Photosynthesis: Mechanisms and Effects, pp 4357–4360. Kluwer Academic Publishers, Dordrecht, The Netherlands
Kramer DM and Crofts AR (1996) Control and measurement of photosynthetic electron transport in vivo. In: Baker NR (ed) Photosynthesis and the Environment, Chapter 2, pp 25–66. Kluwer Academic Publishers, Dordrecht, The Netherlands
Laisk A and Oja V (1994) Range of photosynthetic control of postillumination P700 reduction in sunflower leaves. Photosynth Res 39: 39–50
Malkin S (1968) Kinetic studies on electron-transport components in isolated chloroplasts. I. The effect of the pool of electron carriers between the two photosystems on P700 changes. Biochim Biophys Acta 162: 392–401
Mano J, Miyake C, Schreiber U and Asada K (1995) Photoactivation of the electron flow from NADPH to plastoquinone in spinach chloroplasts. Plant Cell Physiol 36: 1589–1598
Mi H, Endo T, Schreiber U, Ogawa T and Asada K (1995) Thylakoid membrane-bound, NADPH-specific pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 36: 661–668
Mills JD, Crowther D, Walker DA and Hind G (1979a) Electron transport pathways in spinach chloroplasts. Reduction of the primary acceptor of Photosystem II by reduced nicotinamide adenine dinucleotide phosphate in the dark. Biochim Biophys Acta 547: 127–137
Mills JD, Mitchell PD and Barber J (1979b) The cyclic electron transport pathway in chloroplasts. Reduction of plastoquinone by reduced nicotinamide adenine dinucleotide phosphate in the dark. Photobiochem Photobiophys 1: 3–9
Moss DA and Bendall DS (1984) Cyclic electron transport in chloroplasts. The Q-cycle and the site of action of antimycin. Biochim Biophys Acta 767: 389–395
Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M and Shikanai T (2002) PGR5 is involved in cyclic electron flow around Photosystem I and is essential for photoprotection in Arabidopsis. Cell 110: 361–371
Ott T, Clarke J, Birks K and Johnson G (1999) Regulation of the photosynthetic electron transport chain. Planta 209: 250–258
Ryu J-Y, Suh K-H, Chung Y-H, Park Y-M, Chow WS and Park Y-I (2003) NADPH dehydrogenase-mediated respiratory electron transport in thylakoid membranes of the cyanobacterium Synechocystis sp. PCC 6803 is inactive in the light. Mol Cells 15: 240–244
Scheller HV (1996) In vitro cyclic electron transport in barley thylakoids follows two independent pathways. Plant Physiol 110: 187–194
Shikanai T, Endo T, Hashimoto T, Yamada Y, Asada K and Yokota A (1998) Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around Photosystem I. Proc Natl Acad Sci USA 95: 9705–9709
Tagawa K, Tsujimoto HY and Arnon DI (1963) Role of chloroplast ferredoxin in the energy conversion process of photosynthesis. Proc Natl Acad Sci USA 49: 567–572
Wollenberger L, Weibull C and Albertsson P-A (1995) Further characterization of the chloroplast grana margins: the non-detergent preparation of granal Photosystem I cannot reduce ferredoxin in the absence of NADP+ reduction. Biochim Biophys Acta 1230: 10–22
Yao Z-J, Ye J-Y and Mi H-Y (2003) Stimulation of activity of chloroplast NADPH dehydrogenase complex by elevated temperature in tobacco. J Plant Physiol Mol Biol 29: 395–400
Ye JY, Mi JL, Wang YJ and Shen YG (1997) Post-illumination transient increase in chlorophyll fluorescence in spinach thylakoids. Chin Sci Bull 42: 1867–1870 [in Chinese]
Zhang H, Whitelegge JP and Cramer WA (2001) Ferredoxin: NADP+ oxidoreductase is a subunit of the chloroplast cytochrome b 6 f complex. J Biol Chem 276: 38159–38165
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chow, W., Hope, A. Electron Fluxes through Photosystem I in Cucumber Leaf Discs Probed by far-red Light. Photosynthesis Research 81, 77–89 (2004). https://doi.org/10.1023/B:PRES.0000028396.83954.36
Issue Date:
DOI: https://doi.org/10.1023/B:PRES.0000028396.83954.36