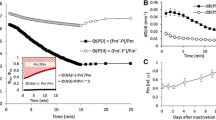
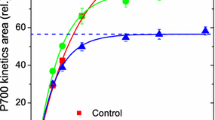
Abstract
Action spectra of photoinactivation of Photosystem II (PS II) in wild-type and chlorophyll b-less barley leaf segments were obtained. Photoinactivation of PS II was monitored by the delivery of electrons from PS II to PS I following single-turnover flashes superimposed on continuous far-red light, the time course of photoinactivation yielding a rate coefficient k i. Susceptibility of PS II to photoinactivation was quantified as the ratio of k i to the moderate irradiance (I) of light at each selected wavelength. k i/I was very much higher in blue light than in red light. The experimental conditions permitted little excess light energy absorbed by chlorophyll (not utilized in photochemical conversion or dissipated in controlled photoprotection) that could lead to photoinactivation of PS II. Therefore, direct absorption of light by Mn in PS II, rather than by chlorophyll, was more likely to have initiated the much more severe photoinactivation in blue light than in red light. Mutant leaves were ca. 1.5-fold more susceptible to photoinactivation than the wild type. Neither the excess-energy mechanism nor the Mn mechanism can explain this difference. Instead, the much lower chlorophyll content of mutant leaves could have exerted an exacerbating effect, possibly partly due to less mutual shading of chloroplasts in the mutant leaves. In general, which mechanism dominates depends on the experimental conditions.





Similar content being viewed by others
Abbreviations
- Chl:
-
Chlorophyll
- E :
-
Excess fraction of absorbed energy
- F :
-
Functional fraction of PS II
- F o, F m :
-
Minimum and maximum Chl fluorescence yield of a dark-adapted leaf, respectively
- \(F_{\text{o}}^{ \prime}\), \(F_{\text{m}}^{ \prime}\), \(F_{\text{s}}^{ \prime}\), \(F_{\text{v}}^{ \prime}\) :
-
Minimum, maximum, steady-state, and variable Chl fluorescence yield of a light-adapted leaf, respectively
- I :
-
Irradiance
- k i :
-
Rate coefficient of photoinactivation of PS II
- LED:
-
Light-emitting diode
- LHCII:
-
Light-harvesting complex II
- P680, P700:
-
Special Chl in the PS II, PS I reaction centre, respectively
- PS:
-
Photosystem
- Q A, Q B :
-
Primary, secondary quinone acceptor in PS II, respectively
- qP :
-
Photochemical quenching coefficient
References
Aro E-M, Virgon I, Andersson B (1993) Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143:113–134
Bodini M, Willis LA, Riechel TL, Sawyer DT (1976) Electrochemical and spectrostudies of manganese (II), -(III), and –(IV) glyconate complexes. 1. Formulas and oxidation-reduction stoichiometry. Inorg Chem 15:1538–1543
Chow WS, Hope AB (2004) Electron fluxes through Photosystem I in cucumber leaf discs probed by far-red light. Photosynth Res 81:77–89
Cser K, Vass I (2007) Radiative and non-radiative charge recombination pathways in Photosystem II studied by thermoluminescence and chlorophyll fluorescence in the cyanobacterium Synechocystis 6803. Biochim Biophys Acta 1767:233–243
Demmig B, Björkman O (1987) Comparison of the effect of excessive light on chlorophyll fluorescence (77 K) and photon yield of O2 evolution in leaves of higher-plants. Planta 171:171–184
Demmig-Adams B, Adams WW III, Barker DH, Logan BA, Bowling DR, Verhoeven AS (1996) Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol Plant 98:253–264
Ettinger WF, Clear AM, Fanning KJ, Peck ML (1999) Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol 119:1379–1385
Ewart AJ (1896) On assimilatory inhibition in plants. J Linn Soc 31:364–461
Hakala M, Tuominen I, Keränen M, Tyystjärvi T, Tyystjärvi E (2005) Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II. Biochim Biophys Acta 1706:68–80
Hideg E, Spetea C, Vass I (1994) Singlet oxygen production in thylakoid membranes during photoinhibition as described by EPR spectroscopy. Photosynth Res 39:191–199
Highkin HS (1950) Chlorophyll studies on barley mutants. Plant Physiol 25:294–306
Hikosaka K, Kato MC, Hirose T (2004) Photosynthetic rates and partitioning of absorbed light energy in photoinhibited leaves. Physiol Plant 121:699–708
Hu Y-Y, Fan D-Y, Losciale P, Chow WS, Zhang W-F (2013) Whole-tissue determination of the rate coefficients of photoinactivation and repair of Photosystem II in cotton leaf discs based on flash-induced P700 redox kinetics. Photosynth Res 117:517–528
Jones LW, Kok B (1966) Photoinhibition of chloroplast reactions. Kinetics and action spectra. Plant Physiol 41:1037–1043
Jung J, Kim H-S (1990) The chromophores as endogenous sensitizers involved in the photogeneration of singlet oxygen in spinach thylakoids. Photochem Photobiol 52:1003–1009
Kato MC, Hikosaka K, Hirotsu N, Makino A, Hirose T (2003) The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in Photosystem II. Plant Cell Physiol 44:318–325
Keren N, Gong H, Ohad I (1995) Oscillations of reaction center II-D1 protein degradation in vivo induced by repetitive light flashes. J Biol Chem 270:806–814
Keren N, Berg A, van Kan PJM, Levanon H, Ohad I (1997) Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: the role of back electron flow. Proc Natl Acad Sci USA 94:1579–1584
Kornyeyev D, Logan BA, Holaday AS (2010) Excitation pressure as a measure of the sensitivity of photosystem II to photoinactivation. Funct Plant Biol 37:943–951
Kou J, Oguchi R, Fan D-Y, Chow WS (2012) The time course of photoinactivation of photosystem II in leaves revisited. Photosynth Res 113:157–164
Lee H-Y, Chow WS, Hong Y-N (1999) Photoinactivation of photosystem II in leaves of Capsicum annuum. Physiol Plant 105:377–384
Lee H-Y, Hong Y-N, Chow WS (2001) Photoinactivation of photosystem II complexes and photoprotection by non-functional neighbours in Capsicum annuum leaves. Planta 212:332–342
Leverenz JW, Öquist G, Wingsle G (1992) Photosynthesis and photoinhibition in leaves of chlorophyll b-less barley in relation to absorbed light. Physiol Plant 85:495–502
Nishiyama Y, Allakhverdiev SI, Murata N (2011) Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol Plant 142:35–46
Oguchi R, Terashima I, Chow WS (2009) The involvement of dual mechanisms of photoinactivation of photosystem II in Capsicum annuum L. plants. Plant Cell Physiol 50:1815–1825
Oguchi R, Terashima I, Kou J, Chow WS (2011a) Operation of dual mechanisms that both lead to photoinactivation of Photosystem II in leaves by visible light. Physiol Plant 142:47–55
Oguchi R, Douwstra P, Fujita T, Chow WS, Terashima I (2011b) Intra-leaf gradients of photoinhibition induced by different color lights: implications for the dual mechanisms of photoinhibition and for the application of conventional chlorophyll fluorometers. New Phytol 191:146–159
Ohad I, Berg A, Berkowicz SM, Kaplan A, Keren N (2011) Photoinactivation of photosystem II: is there more than one way to skin a cat? Physiol Plant 142:79–86
Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N (2005) Two-step mechanism of photodamage to photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 44:8494–8499
Osmond CB (1994) What is photoinhibition? Some insights from comparisons of shade and sun plants. In: Baker NR, Bowyer JR (eds) Photoinhibition of photosynthesis: from molecular mechanisms to the field. BIOS Scientific Publishing Ltd, Oxford, pp 1–24
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—calculation of qP and \(F_{\text{v}}^{\prime}/F_{\text{m}}^{\prime}\;\text{and}\; F_{\text{o}}^{\prime}.\) Photosynth Res 54:135–142
Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol 35:15–44
Santabarbara S, Cazzalini I, Rivadossi A, Garlaschi FM, Zucchelli G, Jenning RC (2002) Photoinhibition in vivo and in vitro involves weakly coupled chlorophyll-protein complexes. Photochem Photobiol 75:613–618
Sarvikas P, Hakala M, Pätsikkä E, Tyystjärvi T, Tyystjärvi E (2006) Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana. Plant Cell Physiol 47:391–400
Schreiber U, Klughammer C (2013) Wavelength-dependent photodamage to Chlorella investigated with a new type of multi-color PAM chlorophyll fluometer. Photosynth Res 114:165–177
Searle GFW, Tredwell CJ, Barber J, Porter G (1979) Picosecond time-resolved fluorescence study of chlorophyll organization and excitation-energy distribution in chloroplasts from wild-type barley and a mutant lacking chlorophyll-b. Biochim Biophys Acta 545:496–507
Tyystjärvi E (2008) Photoinhibition of Photosystem II and photodamage of the oxygen evolving manganese cluster. Coord Chem Rev 252:361–376
Tyystjärvi E, Aro E-M (1996) The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA 93:2213–2218
van Gorkom HJ, Schelvis JPM (1993) Kok’s oxygen clock: what makes it tick? The structure of P680 and consequences of its oxidizing power. Photosynth Res 38:297–301
Vass I (2011) Role of charge recombination processes in photodamage and photoprotection of the photosystem II complex. Physiol Plant 142:6–16
Vass I, Cser K (2009) Janus-faced charge recombinations in photosystem II photoinhibition. Trends Plant Sci 14:200–205
Wei Z, Cady CW, Brudvig GW, Hou HJM (2011) Photodamage of a Mn(III/IV)-oxo mixed-valence compound and photosystem II: evidence that a high-valence manganese species is responsible for UV-induced photodamage of the oxygen-evolving complex in photosystem II. J Photochem Photobiol B 104:118–125
Acknowledgments
The support of this work by a grant awarded to J.H. by the Singapore Millennium Fund (Project code: SMF-Farming System), to WSC by the ARC (DP120100872) and to D.-Y. Fan by the Chinese Academy of Sciences (KZCX2-XB3-09) and the National Natural Science Foundation (31070356) is gratefully acknowledged. W.S.C. values the friendship with Professor Kuang Tingyun that started at a conference in Yangzhou in China in 1984. He wishes Professor Kuang good health and great success in her long-lasting mentoring of young researchers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jie He and Wenquan Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
He, J., Yang, W., Qin, L. et al. Photoinactivation of Photosystem II in wild-type and chlorophyll b-less barley leaves: which mechanism dominates depends on experimental circumstances. Photosynth Res 126, 399–407 (2015). https://doi.org/10.1007/s11120-015-0167-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0167-0