Abstract
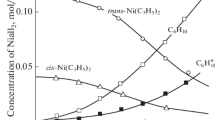
The thermal decomposition of some Ni(II)-carboxylate-imidazole complexes in a nitrogen atmosphere was studied non-isothermally. From the non-isothermal thermoanalytical data, it was found that these complexes decompose through a stepwise release of imidazole molecules and/or CO ones forming unstable intermediates which produce metal oxide or the metal as a final decomposition product. TG in conjunction with DTG were used to evaluate the kinetic and thermodynamic parameters of the decomposition reaction. The kinetic studies were performed employing a computer-oriented kinetic analysis of each set of W-T data obtained under constant heating rate. The diffusion processes are the decisive mechanisms for the decomposition. The values of ΔE, A, ΔH, ΔS and ΔG for activation were calculated for the complexes and correlated to variation in their structure.
Similar content being viewed by others
REFERENCES
H. Sigel, B. E. Fischer and B. Prijs, J. Am. Chem. Soc., 99 (1977) 4489.
J. Reedijk, Recl. Trav. Chim., 88 (1969) 145.
A. Santoro, A. D. Mighel, M. Zocchi and C. W. Reimann, Acta Crystallogr. Sect. B, 25 (1969) 84.
H. M. J. Hendriks and J. Reedijk, Recl. Trav. Chim., 98 (1979) 95.
J. Reedijk and G. C. Verschoor, Acta Crystallogr. Sect. B, 29 (1973) 721.
P. S. Gomm, A. E. Underhill and R. W. A. Oliver, J. Inorg. Nucl. Chem., 34 (1972) 1879.
J. C. Van Dam, G. Hakvoort, J. C. Jansen and J. Reedijk, J. Inorg. Nucl. Chem., 37 (1975) 713.
M. C. Navarro Ranninger and M. Gayoso Andrade, J. Thermal Anal., 14 (1978) 281.
J. G. Van Berkum, G. Hakvoort and J. Reedijk, Thermochim. Acta, 43 (1981) 49.
J. Masson, A. Busnot, F. Busnot, J. F. Hemidy and J. F. Le Querler, Thermochim. Acta, 122 (1987) 221.
R. Curini and S. Materazzi, Thermochim. Acta, 164 (1990) 237.
A. Busnot, J. Masson, J. F. Hemidy and J. F. Querler, Thermochim. Acta, 194 (1992) 265.
W. W. Wendlandt and J. P. Smith, Thermal Properties of Transition Metal Ammine Complexes, Elsevier, Amsterdam 1967.
D. Dollimore, in R. C. MacKenzie (ed.), Differential Thermal Analysis, Academic Press, New York 1970, Chp. 14.
W. W. Wendlandt and L. W. Collins, Thermal Analysis, Dowden-Nutchingol and Ross, 1976, p. 256.
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, 3rd ed., Wiley, New York 1978, p. 331.
J. R. Allan, B. R. Carson, D. L. Gerrard and S. Hoey, Thermochim. Acta, 158 (1990) 91.
J. Kitchen and J. L. Bear, Thermochim. Acta, 1 (1970) 537; J. Inorg. Nucl. Chem., 31 (1969) 2415; 32 (1970) 49.
M. A. Beg and M. A. Qaiser, Thermochim. Acta, 173 (1990) 281.
E. S. Freeman and B. Carroll, J. Phys. Chem., 62 (1958) 394.
A. W. Coats and J. P. Redfern, Nature, 201 (1964) 68.
H. H. Horowitz and G. Metzger, Anal. Chem., 35 (1963) 1464.
J. Zsakó, J. Phys. Chem., 72 (1968) 2406.
J. Zsakó and J. Zsakó, Jr., J. Thermal Anal., 19 (1980) 333.
R. M. Gabr, M. M. Girgis and A. M. El Awad, Thermochim. Acta, 196 (1992) 279.
P. D. Garn, in H. Kambe and P. D. Garn (eds.), Thermal Analysis, Comparative Studies on Materials, Wiley, New York 1974, p. 100.
R. J. Acheson and A. K. Galwey, J. Chem. Soc. A, (1967) 1174.
M. J. McGinn, B. R. Wheeleer and A. K. Galwey, Trans. Faraday Soc., 67 (1971) 1480.
M. J. McGinn, B. R. Wheeler and A. K. Galwey, Trans. Faraday Soc., 66 (1970) 1809.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abd Alla, E.M., Abdel-Hamid, M.I. Kinetics And Mechanism of The Non-isothermal Decomposition. Some Ni(II)-carboxylate-imidazole ternary complexes. Journal of Thermal Analysis and Calorimetry 62, 769–780 (2000). https://doi.org/10.1023/A:1026789828387
Issue Date:
DOI: https://doi.org/10.1023/A:1026789828387