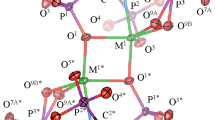
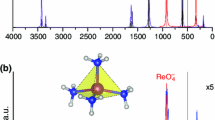
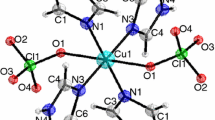
As a result of the interaction of nickel perchlorate with ethylenediamine, imidazole, and semicarbazide, complex compounds Ni(En)3(ClO4)2, Ni(Im)6(ClO4)2, and Ni(SC)3(ClO4)2 have been obtained, the structure and composition of which were confirmed by elemental analysis and by the method of the infrared spectroscopy of frustrated total internal reflection. The kinetics of the thermal decomposition of synthesized compounds in an inert medium was studied within the framework of the traditional method of thermal analysis in a helium atmosphere at a rate of the heating of samples of 5°C/min and under conditions of their high-speed heating (>100°C/min) with simultaneous determination of the composition of gaseous products of thermal decomposition of the material.
Similar content being viewed by others
References
C. de Mello Donegá (Ed.), Nanoparticles: Workhorses of Nanoscience, Springer-Verlag, Berlin (2014).
P. Buffat and J.-P. Borel, Size effect on the melting temperature of gold particles, Phys. Rev. A, 13, No. 6, 2287–2298 (1976).
Z. L. Wang, J. M. Petroski, T. C. Green, and M. A. El-Sayed, Shape transformation and surface melting of cubic and tetrahedral platinum nanocrystals, J. Phys. Chem. B, 102, No. 32, 6145–6151 (1998).
S. G. Vadchenko, M. L. Busurina, E. V. Suvorova, N. I. Mukhina, I. D. Kovalev, and A. E. Sychev, Self-propagating high-temperature synthesis of mechanically activated mixtures in Co–Ti–Al system, Fiz. Goren. Vzryva, 57, No. 1, 58–64 (2021).
O. V. Lapshin and V. G. Prokofev, Mathematical simulation of volumetric and wave gasless combustion in a hybrid mixture of activated and nonactivated powders, Fiz. Goren. Vzryva, 57, No. 4, 80–92 (2021).
U. A. Chumakov and A. G. Knyazeva, Simulation of the synthesis of matrix-inclusions composite materials, Fiz. Goren. Vzryva, 57, No. 1, 93–105 (2021).
Y. R. Parauha, V. Sahu, and S. J. Dhoble, Prospective of combustion method for preparation of nanomaterials: A challenge, Mater. Sci. Eng. B, 267, Article ID 115054 (2021).
W. Zhu, F. Zhao, J. Yao, X. Zhang, H. Wang, C. Xia, and C.-Z. Li, Humic acids as a complexible fuel for combustion synthesis of ceramic nanoparticles, J. Am. Ceram. Soc., 90, No. 12, 4012–4014 (2007).
M. Salehi, M. Galini, M. Kubicki, and A. Khaleghian, Synthesis and characterization of new cobalt (III) and nickel (II) complexes derived from acetylacetone and 2-aminopyridine: A new precursor for preparation of NiO nanoparticles, Russ. J. Inorg. Chem., 64, No. 1, 18–27 (2019).
B. C. Tappan, M. H. Huynh, M. A. Hiskey, D. E. Chavez, E. P. Luther, J. T. Mang, and S. F. Son, Ultralow-density nanostructured metal foams: Combustion synthesis, morphology, and composition, J. Am. Chem. Soc., 128, No. 20, 6589–6594 (2006).
V. V. Boldyrev, R. K. Tukhtaev, A. I. Gavrilov, S. V. Larionov, Z. A. Savel'eva, and L. G. Lavrenova, Combustion of nickel and copper nitrate complexes of hydrazine derivatives as a method for manufacturing fine-grained and porous metals, Russ. J. Inorg. Chem., 43, No. 3, 302–305 (1998).
S. I. Pechenyuk, D. P. Domonov, and A. N. Gosteva, Thermal decomposition of cationic, anionic, and binary complex compounds of 3d metals, Ros. Khim. Zh., LXIV, No. 1, 45–69 (2020).
P. Afanasiev, S. Chouzier, T. Czeri, G. Pilet, C. Pichon, M. Roy, and M. Vrinat, Cobalt hexamethylentetramine complexes (NO3)2Me(H2O)6(HMTA)2∙4H2O (Me = Co2+, Ni2+): New molecular precursors for the preparation of metal, J. Inorg. Chem., 47, No. 7, 2303–2311 (2008).
B. N. Sivasankar and L. Ragunath, Thermal degradation kinetics of Co(II), Ni(II), and Zn(II) hydrazinesulfinates in air, oxygen and nitrogen atmospheres, Thermochimica Acta., 397, 237–247 (2003).
A. I. Gavrilov, R. K. Tukhtaev, S. V. Larionov, L. G. Lavreneva, Z. A. Savel'eva, and V. V. Boldyrev, Production of finely divided nickel with the controlled morphology in combustion, Dokl. Fiz. Khim., 348, No. 2, 104–107 (1996).
G. S. Smith, Co-ordination compounds of semicarbaxide, phenylsernicarbaxide, m-tolybemimrbazide, and aminoguartidine, J. Chem. Soc., 1354–1358 (1937).
O. P. Korobeinichev, A. S. Shmelev, V. G. Voronov, and G. I. Anisiforov, Application of dynamic mass spectrometry and computers for kinetic study. Construction of a kinetic model and determination of kinetic constants in thermal decomposition reactions. Therm. Analysis, 1, 77–83 (1975).
O. P. Korobeinichev, L. V. Kuibida, A. A. Paletsky, and A. G. Shmakov, Molecular-beam mass-spectrometry to ammonium dinitramide combustion chemistry studies, J. Propuls. Power, 14, No. 6, 991–1000 (1998).
O. P. Korobeinichev, S. A. Trubachev, A. K. Joshi, A. Kumar, A. A. Paletsky, A. G. Tereshchenko, A. G. Shmakov, R. K. Glaznev, V. Raghavan, and A. M. Mebel, Experimental and numerical studies of downward flame spread over PMMA with and without addition of tri phenyl phosphate, in: Proc. Combust. Inst., 38, 4867–4875 (2021).
NIST Standard Reference Database Number 69; https://doi.org/10.18434/T4D303https://webbook.nist.gov/chemistry.
J. B. Hodgson, G. C. Percy, and D. A. Thornton, The infrared spectra of imidazole complexes of first transition series metal(ii) nitrates and perchlorates, J. Mol. Struct., 66, 81–92 (1980).
W. J. Eilbeck, F. Holmes, and A. E. Underhill, Cobalt(II), nickel(II), and copper(II) complexes of imidazole and thiazole, J. Chem. Soc. A, 757–761 (1967).
R. Ramasamy, Vibrational spectroscopic studies of imidazole, Armen. J. Phys., 8, No. 1, 51–55 (2015).
B. Morzyk-Ociepa, E. R6życka-Sokołowska, and D. Michalska, Revised crystal and molecular structure, FT-IR spectra and DFT studies of chlorotetrakis(imidazole)copper(II) chloride, J. Mol. Struct., 1028, 49–56 (2012).
K. Krishnan and R. A. Plane, Raman and infrared spectra of complexes of ethylenediamine with zinc(II), cadmium(II), and mercury(II), Inorg. Chem., 5, No. 5, 852–857 (1966).
G. W. Watt, J. T. Summers, E. M. Potrafke, and E. R. Birnbaum, Deprotonation of tris (ethylenediamine)osmium halides, Inorg. Chem., 5, No. 5, 857–860 (1966).
M. A. Sarukhanov, S. A. Slivko, and Z. K. Kamalov, Electronic structure and vibrational spectrum of semicarbazide, J. Struct. Chem., 33, 651–656 (1992).
S. V. Kasmir Raja, G. A. Savariraj, and D. N. Sathyanarayana, Vibrational spectra and normal coordinates for semicarbazide and semicarbazide hydrochloride, Ind. J. Chem., 18 A, 297–301 (1979).
S. K. Amini, N. L. Hadipour, and F. Elmi, A study of hydrogen bond of imidazole and its 4-nitro derivative by ab initio and DFT calculated NQR parameters, Chem. Phys. Lett., 391, Nos. 1–3, 95–100 (2004).
B.-D. Wu, S.-W. Wang, L. Yang, T.-L. Zhang, J.-G. Zhang, and Z.-N. Zhou, Preparation, crystal structure, and thermal decomposition of two novel energetic compounds [Ni(IMI)6](L)2 (\( \textrm{L}={\textrm{ClO}}_4^{-} \) and \( {\textrm{NO}}_3^{-} \)) and one carbonate compound [Ni(IMI)6](CO3)∙5H2O (IMI=imidazole), Z. Anorg. Allg. Chem., 637, Nos. 14–15, 2252–2259 (2011).
L. N. Swink and M. Atoji, The crystal structure of triethylenediamine-nickel(II)nitrate, Ni(NH2CH2CH2NH2)3(NO3)2, Acta Cryst., 13, 639–643 (1960).
G. Brewer, R. J. Butcher, and J. P. Jasinski, Tris(ethane-1,2-diamine-[kappa]2N,N′)nickel (II) diiodide, Acta Cryst., 66, 103–104 (2010).
J. Y. Guo, G. X. Ma, T. L. Zhang, J.-G. Zhang, and Y.-H. Liu, Study on two coordination compounds using semicarbazide (SCZ) as bidentate ligand: [Ni(SCZ)3](NO3)2 and Cu(SCZ)2Cl2, Trans. Met. Chem., 32, 413–418 (2007).
M. C. N. Ranninger, M. G. Andrade, and M. A. A. Franco, Thermal decomposition of some imidazole and N-methyl substituted imidazole complexes of palladium (II), J. Therm. Anal., 14, 281–290 (1978).
T. D. George and W. W. Wendlandt, The thermal decomposition of metal complexes. II Some ammine and ethylenediamine complexes of nickel (II), J. Inorg. Nucl. Chem., 25, No. 4, 395–405 (1963).
I. E. House and F. M. Tahir, Deamination of tris(ethylenediamine)nickel(II) chloride and tris(ethylenediamine) platinum(IV) chloride, Thermochimica Acta., 118, 191–197 (1987).
G. Singh, S. P. Felix, and D. K. Pandey, Studies on energetic compounds part 37: Kinetics of thermal decomposition of perchlorate complexes of some transition metals with ethylenediamine, Thermochim. Acta., 411, No. 1, 61–71 (2004).
H. H. Horowitz and G. Metzger, A new analysis of thermogravimetric traces, Anal. Chem., 35, No. 10, 1464–1468 (1963).
A. Coats and J. Redfern, Kinetic parameters from thermogravimetric data, Nature, 201, 68–69 (1964).
E. S. Freeman and B. Carroll, The application of thermoanalytical techniques to reaction kinetics: The thermogravimetric evaluation of the kinetics of the decomposition of calcium oxalate monohydrate, J. Phys. Chem., 62, No. 4, 394–397 (1958).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Inzhenerno-Fizicheskii Zhurnal, Vol. 95, No. 7, pp. 1780–1793, November–December 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shmakov, A.G., Paletskii, A.A., Komova, O.V. et al. Kinetic Laws Governing Thermal Decomposition of Perchlorate Nickel Organometallic Complexes Under Changes of the Ligand Nature. J Eng Phys Thermophy 95, 1732–1745 (2022). https://doi.org/10.1007/s10891-022-02644-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10891-022-02644-2