Abstract
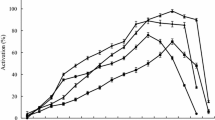
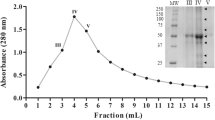
Asclepias fruticosa L. is a small shrub containing latex with proteolytic activity. The crude extract (latex diluted 1:250 and ultracentrifuged) contained 276 μg of protein/mL and the proteolytic activity reached 1.2 caseinolytic U/mL. This enzyme preparation was very stable even after 2 hours at 45°C, but was quickly inactivated after 5 minutes at 80°C. Chromatographic purification was achieved by FPLC using a cation exchanger (SP-Sepharose FF). Thus, a unique proteolitically active fraction could be isolated, being homogeneous by bidimensional electrophoresis and mass spectrometry (Mr = 23,652). The optimum pH range was achieved at 8.5–10.5. The enzyme activity was completely inhibited by specific cysteine peptidases inhibitors. Isoelectric focusing followed by zymogram showed the enzyme had a pI greater than 9.3. The N-terminus sequence (LPDSVDWREKGVVFPIRNQGK) shows a great deal of similarity to those of the other cysteine endopeptidases isolated from latices of Asclepiadaceae even when a high degree of homology could be observed with other plant cysteine endopeptidases.
Similar content being viewed by others
REFERENCES
Abraham, K. I. and Joshi, P. N. (1979a). Biochim.Biophys.Acta 568, 111–119.
Abraham, K. I. and Joshi, P. N. (1979b). Biochim.Biophys.Acta 568, 120–126.
Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D. J. (1997). Nucleic Acids Res. 25, 3389–3402.
Arribére, M. C., Cortadi, A. A., Gattuso, M. A., Bettiol, M. P., Priolo, N. S., and Caffini, N. O. (1998). Phytochem.Anal. 9, 267–273.
Barragán, B. E., Hernández-Arana, A., Oliver, M., Castaneda-Agulló, M., and Del Castillo, L. M. (1985). Rev.Latinoamer.Quim.16, 158–160.
Barrett, A. J. and Kirschke, H. (1981). Meth.Enzymol. 80, 535–561.
Bose, S. M. and Madhavakrishna, W. (1958). Enzymologia 19, 186–200.
Bradford, M. M. (1976). Anal.Biochem. 72, 248–254.
Brockbank, W. J. and Lynn, K. R. (1979). Biochim.Biophys.Acta 578, 13–22.
Choi, K. H., Laursen, R. A., and Allen, K. N. (1999). Biochemistry 38, 11624–11633.
Cohen, L. W., Coghlan, V. M., and Dihel, L. C. (1986). Gene 48, 219–227.
Dubois, T., Kleinschmidt, T., Schnek, A. G., Looze, Y., and Braunitzer, G. (1988). Biol.Chem.Hoppe-Seyler 369, 741–754.
Endress, M. E. and Bruyns, P. V. (2000). Bot.Rev. 66, 1–56.
Firsthoefel, N. R., Cushman, M. A. F., Ostrem, J. A., and Cushman, J. C. (1998). Plant Sci. 136, 195–206.
Gan, S. and Amasino, R. M. (1995). Science 270, 1986–1988.
Good, N. E. and Izawa, S. (1972). Meth.Enzymol. 24, 53–68.
Greenberg, D. M. and Winnick, T. (1940). J.Biol.Chem. 135, 761–787.
Griffiths, C. M., Hosken, S. E., Oliver, D., Chojecki, J., and Thomas, H. (1997). Plant Mol.Biol. 34, 815–821.
Guerrero, F. D., Jones, J. T., and Mullet, J. E. (1990). Plant Mol.Biol. 15, 11–26.
Jaziri, M., Kleinschmidt, T., Walraevens, V., Schnek, A. G., and Looze, Y. (1994). Biol.Chem.Hoppe-Seyler 375, 379–385.
Jones, M. L., Larsen, P. B., and Woodson, W. R. (1995). Plant.Mol. Biol. 28, 505–512.
Laemmli, U. K. (1970). Nature 227, 680–685.
López, L. M. I. (1995). “Aislamiento, purificación y caracterización de proteasas del látex de Maclura pomifera (Raf.) Schneid. (Moraceae).” Ph. D. thesis, Faculty of Exact Sciences, La Plata University, Argentina.
López, L. M. I., Sequeiros, C., Natalucci, C. L., Brullo, A., Maras, B., Barra, D., and Caffini, N. (2000). Protein Express.Purif. 18, 133–140.
Lynn, K. R., Brockbank, W. J., and Clevette, N. A. (1980a). Biochim. Biophys.Acta 612, 119–125.
Lynn, K. R., Yaguchi, M., and Roy, C. (1980b). Biochim.Biophys. Acta 624, 579–580.
Madhavakrishna, W. and Bose, S. M. (1960). Enzymologia 22, 251–261.
Noh, Y. S. and Amasino, R. M. (1999). Plant Mol.Biol. 41, 195–206.
Obregón, W. D., Arribére, M. C., Morcelle del Valle, S., Liggieri, C., Caffini, N. O., and Priolo, N. S. (2001). J.Protein Chem. 20, 317–325.
Pal, G. and Sinha, N. K. (1980). Arch.Biochem.Biophys. 202, 321–329.
Priolo, N., Morcelle del Valle, S., Arribére, M. C., López, L. M. I., and Caffini, N. O. (2000). J.Protein Chem. 19, 39–49.
Priolo, N., Arribére, M. C., Caffini, N., Barberis, S., Nieto Vázquez, R., and Luco, J. (2001). J.Mol.Catal.B: Enzymatic 635, 1–13.
Ritonja, A., Buttle, D. J., Rawlings, N. D., Turk, V., and Barrett, A. J. (1989). FEBS Lett. 258, 109–112.
Rodriquez-Romero, A. and Hernández-Arana, A. (1998). In Handbook of Proteolytic Enzymes (Barrett, A. J., Rawlings, N. D., and Woessner, J. F., eds.), Academic Press, London, pp. 576–578.
Rowan, A. D. and Buttle, D. J. (1994). Meth.Enzymol. 244, 555–568.
Salvesen, G. and Nagase, H. (1989). In Proteolytic enzymes: A practical approach (Beynon, R. J. and Bond, J. S., eds.), Oxford, United Kingdom: IRL Press, pp. 83–104.
Schaffer, M. A. and Fischer, R. L. (1988). Plant Physiol. 87, 431–436.
Sengupta, A., Bhattacharya, D., Pal, G., and Sinha, N. K. (1984). Arch. Biochem.Biophys. 232, 17–25.
Tablero, M., Arreguín, R., Arreguín, B., Soriano, M., Sánchez, R. I., Rodriguez-Romero, A., and Hernández-Arana, A. (1991). Plant Sci. 74, 7–15.
Tournaire, C., Kushnir, S., Bauw, G., Inze, D., Teyssendier de la Serve, B., and Renaudin, J. P. (1996). Plant Physiol. 111, 159–168.
Tranbarger, T. J. and Misra, S. (1996). Gene 172, 221–226.
Ueda, T., Seo, S., Ohashi, Y., and Hashimoto, J. (2000). Plant Mol. Biol. 44, 649–657.
Utsumi, S., Damodaran, S., and Kinsella, J. E. (1984). J.Agric.Food Chem. 32, 1406–1412.
Vairo Cavalli, S. E., Cortadi, A., Arribére, M. C., Conforti, P., Caffini, N. O., and Priolo, N. S. (2001). Biol.Chem.Hoppe-Seyler 382, 879–883.
Watanabe, H., Abe, K., Emori, Y., Hosoyama, H., and Arai, S. (1991). J.Biol.Chem. 266, 16897–16902.
Watson, D. C., Yaguchi, M., and Lynn, K. R. (1990). Biochem.J. 266, 75–81.
Westergaar, J. L., Hackbarth, C., Treuhaft, M. W., and Roberts, R. C. (1980). J.Immunol.Meth. 34, 167–175.
Winnick, T., Davies, A. R., and Greenberg, D. M. (1940). J.Gen. Physiol. 23, 275–308.
Ye, Z. H. and Varner, J. E. (1996). Plant Mol.Biol. 30, 1233–1246.
Yu, W. J. and Greenwood, J. S. (1994). Exp.Bot. 45, 261–268.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trejo, S.A., López, L.M.I., Cimino, C.V. et al. Purification and Characterization of a New Plant Endopeptidase Isolated from Latex of Asclepias fruticosa L. (Asclepiadaceae). J Protein Chem 20, 469–477 (2001). https://doi.org/10.1023/A:1012502412612
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1012502412612