Abstract
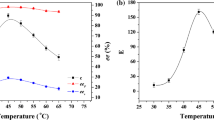
The acetylation of 3-phenylthio-2-propanol (168 mg) was performed with vinyl acetate (1 ml) using different lipases from 15 °C to 51 °C. As a result, the (R)-enantiomer was selectively acetylated and the (S)-enantiomer was non-reactive in all the cases. An appropriate choice of conditions can be made to isolate both (R)-alcohol (ee 99%, 36 h, conversion 46%, sub/enz: 1/2) and (S)-alcohol (ee 93%, 38 h, conversion 46%, THF, sub/enz: 1 l−1) using Humicola lanuginosalipase (Lipolase). Increasing the amount of enzyme increased the ee.
Similar content being viewed by others
References
Crumbie RL, Deol BS, Nemorin JE, Ridley DD (1978) Asymmetric reduction of carbonyl compounds by yeast. IV: Preparation of optically active β-hydroxy sulfides, sulfoxides and sulfones. Aust. J. Chem. 31: 1965-1980.
Drauz K, Waldmann H (1995) Enzyme Catalysis in Organic Synthesis. Weinheim: VCH.
Fujisawa T, Itoh T, Nakai M, Sato T (1985) Optically pure (S)-3-phenylthio-1, 2-propanediol: synthesis by the yeast reduction and use as a precursor of both enantiomers of secondary alcohols. Tetrahedron Lett. 26: 771-774.
Jaeger KE, Reetz MT (1998) Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 16: 396-403.
Koskinen AMP, Klibanov AM, eds. (1996) Enzymatic Reactions in Organic Media, Glasgow: Blackie A & P.
Miyazawa T, Yukawa T, Ueji S, Yanagihara R, Yamada T (1998) Resolution of 2-phenoxy-1-propanols by Pseudomonassp. lipase-catalyzed highly enantioselective transesterification: influence of reaction conditions on the enantioselectivity towards primary alcohols. Biotechnol. Lett. 20: 235-238.
Tuomi WV, Kazlauskas RJ (1999) Molecular basis for enantioselectivity of lipase from Pseudomonas cepaciatowards primary alcohols. modeling, kinetics and chemical modification of Tyr29 to increase or decrease enantioselectivity. J. Org. Chem. 64: 2638-2647.
Whitesell JK, Reynolds D (1983) Resolution of chiral alcohols with mandelic acid. J. Org. Chem. 48: 3548-3551.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Singh, S., Kumar, S. & Chimni, S.S. Enantioselective resolution of 3-phenylthio-2-propanol with Humicola lanuginosa lipase. Biotechnology Letters 22, 1237–1241 (2000). https://doi.org/10.1023/A:1005609332450
Issue Date:
DOI: https://doi.org/10.1023/A:1005609332450