Abstract
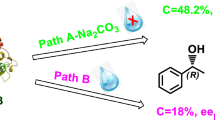
Stereoselective hydrolysis of (±)-2-(4-hydroxyphenyl)propionic acid ethyl ester (2-HPPAEE) by lipase catalyzed in aqueous system was investigated. Lipase AK with higher catalytic activity and enantioselectivity was selected as catalyst. Simultaneously, factors affecting the conversion of substrate (c) and the enantiomeric excess of product (eep) were optimized. The optimal conditions were established, involving 45 °C of temperature, 5.5 of pH, 10 mg of lipase AK dosage, 0.04 mmol of substrate dosage and 40 h of reaction time. Under the optimum conditions, c and eep could reach up to 49% and 98%, respectively.







Similar content being viewed by others
References
Arpigny JL, Jaeger KE (1999) Bacterial lipolytic enzymes: classification and properties. Biochem J 343:177–183. https://doi.org/10.1042/bj3430177
Cho SH, Wang PY, Tsai SW (2011) Lipase-catalyzed hydrolytic resolution of (+,−)-3-hydroxy-3-phenylpropionates in biphasic media. J Taiwan Inst Chem E 42:408–412. https://doi.org/10.1016/j.jtice.2010.09.005
Dong HP, Wang YJ, Zheng YG (2010) Enantioselective hydrolysis of diethyl 3-hydroxyglutarate to ethyl (S)-3-hydroxyglutarate by immobilized Candida antarctica lipase. J Mol Catal B-Enzym 66:90–94. https://doi.org/10.1016/j.molcatb.2010.03.009
Erdemir S, Yilmaz M (2012) Catalytic effect of calix [n] arene based sol–gel encapsulated or covalent immobilized lipases on enantioselective hydrolysis of (R/S)-naproxen methyl ester. J Incl Phenom Macro Chem 72:189–196. https://doi.org/10.1007/s10847-011-9962-1
Ghanem A, Aboul-Enein HY (2004) Lipase-mediated chiral resolution of racemates in organic solvents. Tetrahedron Asymmetry 15:3331–3351. https://doi.org/10.1016/j.tetasy.2004.09.019
Gilani SL, Najafpour GD, Heydarzadeh HD, Moghadamnia A (2017) Enantioselective synthesis of (S)-naproxen using immobilized lipase on chitosan beads. Chirality 29:304–314. https://doi.org/10.1002/chir.22689
Hu C, Wang N, Zhang W et al (2015) Immobilization of Aspergillus terreus lipase in self-assembled hollow nanospheres for enantioselective hydrolysis of ketoprofen vinyl ester. J Biotechnol 194:12–18. https://doi.org/10.1016/j.jbiotec.2014.11.032
Leśniarek A, Gładkowski W, Chojnacka A (2018) Application of Lecitase® Ultra-catalyzed Hydrolysis to the Kinetic resolution of (E)-4-phenylbut-3-en-2-yl esters. Catalysts 8:423. https://doi.org/10.3390/catal8100423
Li N, Hu SB, Feng GY (2012) Resolution of 2-nitroalcohols by Burkholderia cepacia lipase-catalyzed enantioselective acylation. Biotechnol Lett 34:153–158. https://doi.org/10.1007/s10529-011-0754-x
Lv CG, Jia GF, Zhu WT et al (2015) Enantiomeric resolution of new triazole compounds by high-performance liquid chromatography. J Sep Sci 30:344–351. https://doi.org/10.1002/jssc.200600282
Marciello M, Filice M, Palomo JM (2012) Different strategies to enhance the activity of lipase catalysts. Catal Sci Technol 2:1531–1543. https://doi.org/10.1039/c2cy20125a
Miyazawa T, Iguchi W (2013) Long-chain ethers as solvents can amplify the enantioselectivity of theCarica papayalipase-catalyzed transesterification of 2-(substituted phenoxy)propanoic acid esters. Biotechnol Lett 35:1639–1643. https://doi.org/10.1007/s10529-013-1247-x
Neumann H, Brennführer A, Beller M (2010) An efficient and practical sequential one-pot synthesis of suprofen, ketoprofen and other 2-arylpropionic acids. Adv Synth Catal 350:2437–2442. https://doi.org/10.1002/adsc.200800415
Palomo JM, Fuentes M, Fernández-Lorente G, Mateo C, Guisan JM, Fernández-Lafuente R (2003) General trend of lipase to self-assemble giving bimolecular aggregates greatly modifies the enzyme functionality. Biomacromolecules 4:1–6. https://doi.org/10.1021/bm025729+
Saric M, van der Wielen LAM, Straathof AJJ (2011) Theoretical performance of countercurrent reactors for production of enantiopure compounds. Chem Eng Sci 66:510–518. https://doi.org/10.1016/j.ces.2010.11.020
Schuur B, Verkuijl BJ, Minnaard AJ, de Vries JG, Heeres HJ, Feringa BL (2011) Chiral separation by enantioselective liquid-liquid extraction. Org Biomol Chem 9:36–51. https://doi.org/10.1039/c0ob00610f
Shen J, Okamoto Y (2015) Efficient separation of enantiomers using stereoregular chiral polymers. Chem Rev 116:1094–1138. https://doi.org/10.1021/acs.chemrev.5b00317
Siodmiak T, Mangelings D, Vander Heyden Y, Ziegler-Borowska M, Marszall MP (2015) High enantioselective Novozym 435-catalyzed esterification of (R,S)-flurbiprofen monitored with a chiral stationary phase. Appl Biochem Biotechnol 175:2769–2785. https://doi.org/10.1007/s12010-014-1455-4
Takaç S, Mutlu D (2007) A parametric study on biphasic medium conditions for the enantioselective production of naproxen by Candida rugosa lipase. Appl Biochem Biotechnol 141:15–26. https://doi.org/10.1007/s12010-007-9207-3
Tang KW, Zhang PL, Pan C (2011) Experimental and simulation on enantioselective extraction in centrifugal contactor separators. AIChE J 57:3027–3036. https://doi.org/10.1002/aic.14004
Tong S, Wang X, Lu M, Xiong Q, Wang Q, Yan J (2016) Enantioseparation of 2-(substituted phenyl) propanoic acids by high-speed countercurrent chromatography and investigation of the influence of substituents in enantiorecognition. J Sep Sci 39:1567–1573. https://doi.org/10.1002/jssc.201600171
Ushiyama M, Furuya T (1989) Biotransformation of (RS)-tropic acid in suspension cultures of Coffea arabica, Datura innoxia, Eucalyptus perriniana and Nicotiana tabacum. Phytochemistry. https://doi.org/10.1016/S0031-9422(00)97979-2
Vetter T, Burcham CL, Doherty MF (2015) Separation of conglomerate forming enantiomers using a novel continuous preferential crystallization process. AIChE J 61:2810–2823. https://doi.org/10.1002/aic.14934
Wacharineantar S, Levilain G, Dupray V et al (2010) Resolution of (±)-Imeglimin-2,4-dichlorophenylacetate methanol solvate by preferential crystallization. Org Process Res Dev 14:1358–1363. https://doi.org/10.1021/op100173r
Weng X, Baez JE, Khiterer M, Hoe MY, Bao Z, Shea KJ (2015) Chiral polymers of intrinsic microporosity: selective membrane permeation of enantiomers. Angew Chem Int Ed 54:11214–11218. https://doi.org/10.1002/ange.201504934
Ye J, Yu W, Chen G et al (2010) Enantiomeric separation of 2-arylpropionic acid nonsteroidal anti-inflammatory drugs by HPLC with hydroxypropyl-β-cyclodextrin as chiral mobile phase additive. Biomed Chromatogr 24:799–807. https://doi.org/10.1002/bmc.1365
Yilmaz E, Can K, Sezgin M et al (2011) Immobilization of Candida rugosa lipase on glass beads for enantioselective hydrolysis of racemic Naproxen methyl ester. Bioresour Technol 102:499–506. https://doi.org/10.1016/j.biortech.2010.08.083
Zhang PL, Cheng Q, Xu W et al (2019) Enzymatic enantioselective hydrolysis of 2-(3-chlorophenyl) propionic acid ester enhanced by PEG: experiment and optimization. Ind Eng Chem Res 57:11246–11256. https://doi.org/10.1021/acs.iecr.8b02377
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant numbers, 21676077).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, X., Zhang, P., Liu, G. et al. Lipase-catalyzed hydrolysis of 2-(4-hydroxyphenyl)propionic acid ethyl ester to (R)-(−)-2-(4-hydroxyphenyl)propanoic acid. Chem. Pap. 73, 2461–2468 (2019). https://doi.org/10.1007/s11696-019-00796-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00796-9