Abstract
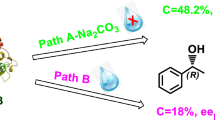
The use of fatty and aromatic acids as green acyl donors for enzymatic kinetic resolution via esterification of 1-phenylethanol and 1-phenylpropan-2-ol was described. The impact of the presence of magnesium sulfate on both reactivity and selectivity of Candida rugosa lipase (CRL) was checked. The organic solvents, the medium dilution, and the temperature revealed as determinant parameters to achieve enantioselective esterification reactions. A significant impact of the use of magnesium sulfate was revealed on the enantioselectivity of the CRL in heptane during the resolution of 1-phenylethanol, using butyric and lauric acids as acyl donors.
Graphical abstract


Similar content being viewed by others
Data availability
The authors confirm that all data supporting this article is available within the main manuscript and the supporting information.
References
Gerlach D, Schreier P (1989) Biocatalysis 2:257
Chua LS, Sarmidi MR (2006) Enzyme Microb Technol 38:551
Karadeniz F, Bayraktar E, Mehmetoglu Ü (2010) Artif Cells, Blood Substitutes Biotechnol 38:288
Paravidino M, Hanefeld U (2011) Green Chem 13:2651
Sikora A, Siódmiak T, Marszałł MP (2014) Chirality 26:663
Brodzka A, Koszelewski D, Zysk M, Ostaszewski R (2018) Catal Commun 106:82
Ali SH, Tarakmah A, Merchant SQ, Al-Sahhaf T (2007) Chem Eng Sci 62:3197
Fan C, Wan R, Kong P, Wang X, Wang J, Zhang X, Zheng Z (2020) Catal Commun 140:106002
Khan Z, Javed F, Shamair Z, Hafeez A, Fazal T, Aslam A, Rehman F (2021) J Ind Eng Chem 103:80
Laboret F, Perraud R (1999) Appl Biochem Biotechnol 82:185
Alvarez E, Rodriguez J, Villa R, Gomez C, Nieto S, Donaire A, Lozano P (2019) ACS Sustainable Chem Eng 7:13307
Bayout I, Bouzemi N, Guo N, Mao X, Serra S, Riva S, Secundo F (2020) Flavour Fragrance J 35:209
Gamayurova VS, Zinoveva ME, Shnaider KL, Davletshina GA (2021) Catal Ind 13:58
Chua LS, Sarmidi M (2004) J Mol Catal B: Enzym 28:111
Vanin AB, Orlando T, Piazza SP, Puton BM, Cansian RL, Oliveira D, Paroul N (2014) Appl Biochem Biotechnol 174:1286
Lortie R (1997) Biotechnol Adv 15:1
Zieniuk B, Fabiszewska A, Wołoszynowska M, Białecka-Florjańczyk E (2021) Biocatal Biotransform 39:455
Bôas V, de Castro HF (2021) Biotechnol Bioeng 119:725
Irimescu R, Saito T, Kato K (2003) J Am Oil Chem Soc 80:659
Gawas SD, Jadhav SV, Rathod VK (2016) App Biochem Biotechnol 180:1428
Cea M, González ME, Abarzúa M, Navia R (2019) J Environ Manage 242:171
Hari Krishna S, Karanth NG (2007) Catal Rev 44:499
Faber K (2011) Biotransformations in Organic Chemistry, 6th edn. Springer-Verlag, Berlin
Simić S, Zukić E, Schmermund L, Faber K, Winkler CK, Kroutil W (2021) Chem Rev 122:1052
Merabet-Khelassi M, Aribi-Zouioueche L, Riant O (2008) Tetrahedron 19:2378
Merabet-Khelassi M, Bouzemi N, Fiaud JC, Riant O, Aribi-Zouioueche L (2011) C R Chim 14:978
Stergiou PY, Foukis A, Filippou M, Koukouritaki M, Parapouli M, Theodorou LG, Hatziloukas E, Afendra A, Pandey A, Papamichael EM (2013) Biotechnol Adv 31:1846
Lajtai-Szabó P, Nemestóthy N, Gubicza L (2020) Hung J Ind Chem 48:9
Bezbradica D, Crovic MJ, Tanaskovic S, Lukovic N, Carevic M, Milivojevic A, Knezevic-Jugovic Z (2017) Curr Org Chem 21:104
Duan Y, Du Z, Yao Y, Li R, Wu D (2006) J Agric Food Chem 54:6219
Silva GS, Santos JC, Oliveira PC, De Castro HF (2011) Eur Food Res Technol 233:743
Findrik Z, Németh G, Vasić-Rački Đ, Bélafi-Bakó K, Csanádi Z, Gubicza L (2012) Proc Biochem 47:1715
Freitas L, Zieniuk B, Groborz K, Wołoszynowska M, Ratusz K, Białecka-Florjańczyk E, Fabiszewska A (2021) Biomolecules 11:314
Benamara NE, Merabet-Khelassi M, Lakoud SG, Aribi-Zouioueche L, Riant O (2021) ChemistrySelect 6:13941
Kagan HB, Fiaud JC (1988) Kinetic Resolution. In: Eliel EL, Wilen SH (eds), Topics in Stereochemistry, vol 18. Wiley & Sons, New York
Chen CS, Fujimoto Y, Sih CJ (1982) J Am Chem Soc 104:7294
Hayashi T, Matsumoto Y (1991) Tetrahedron 2:601
Naemura K, Murata M, Tanaka R, Yano M, Hirose K, Tobe Y (1996) Tetrahedron 7:1581
Naemura K, Murata M, Tanaka R, Yano M, Hirose K, Tobe Y (1996) Tetrahedron 7:3285
Kazlauskas RJ, Weissfloch AN, Rappaport AT, Cuccia LA (1991) J Org Chem 56:2656
Okamoto T, Ueji S (2000) Biotechnol Lett 22:1169
Salgin S, Takaç S (2007) Chem Eng Technol 30:1739
Cowan JA (2002) Biometals 15:225
Tran-Ha MH, Santos V, Wiley DE (2005) J Membrane Sci 251:179
Merabet-Khelassi M, Houiene Z, Aribi-Zouioueche L, Riant O (2012) Tetrahedron 23:828
Frings K, Koch K, Hatmeier W (1999) Enzyme Microb Technol 25:303
Ottoson J, Hult K (2001) J Mol Catal B 11:1025
Suan C, Sarmidi MR (2004) J Mol Catal B 28:111
Janssen AE, Vaidya AM, Halling PJ (1996) Enz Microb Technol 18:340
Pleiss J, Fischer M, Schmid RD (1998) Chem Phys Lipids 93:67
Razi S, Zeror S, Merabet-Khelassi M, Kolodziej E, Toffano M, Aribi-Zouioueche L (2021) Catal Lett 151:2603
Melais N, Aribi-Zouioueche L, Riant O (2016) C R Chimie 19:971
Acknowledgements
Algerian Ministry of Higher Education and Scientific Research (MESRS, FNR 2000) and ANDRU (PNR) are gratefully acknowledged for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smaine, F.Z., Merabet-Khelassi, M., Zeror, S. et al. Fatty and aromatic acids as acyl donors in enzymatic kinetic resolution of phenylethanol and 1-phenylpropan-2-ol. Monatsh Chem 155, 105–113 (2024). https://doi.org/10.1007/s00706-023-03147-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03147-3