Abstract
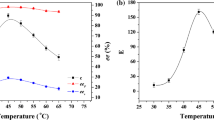
The enantioselectivity for lipase-catalyzed esterifications of 2-(4-substituted phenoxy)propionic acids in organic solvents was found to be mainly controlled by both size (steric) and electronic effects of substituents: H, F, Cl, CF3 and CH3. For the similar substituents in size, CF3 and CH3, however, their electronic effects play an important role in controlling the enantioselectivity. A model for the enantiorecognition is proposed by the discussion based on the value of the Michaelis constant obtained for the enantiomers.
Similar content being viewed by others
References
Chen C-S, Fujimoto Y, Girdaukas G, Sih CJ (1982) Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 104: 7294–7299.
Chen C-S, Sih CJ (1989) General aspects and optimization of enantioselective biocatalysis in organic solvents. The use of lipase. Angew. Chem. Int. Ed. Engl. 28: 695–707.
Dahod SK, S-Mangano P (1987) Carbon tetrachloride-promoted stereo selective hydrolysis of methyl-2-chloropropionate by lipase. Biotech. Bioeng. 30: 995–999.
Dordick JS (1989) Enzymatic catalysis in monophasic organic solvents. Enz. Microb. Technol. 11: 194–211.
Fitzpatrick PA, Klibanov AM (1991) How can the solvent affect enzyme enantioselectivity? J. Am. Chem. Soc. 113: 3166–3171.
Jaffe HH (1953) A reexamination of the Hammett equation. Chem. Rev. 53: 191–261.
Kanerva LT, Klibanov AM (1989) Hammett analysis of enzyme action in organic solvents. J. Am. Chem. Soc. 111: 6864–6865.
Kazlauskas RJ, Weissfloch ANE, Rappaport AT, Cuccia LA (1991) A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalyzed by cholesterol esterase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J. Org. Chem. 56: 2656–2665.
Kitaguchi H, Itoh I, Ono M (1990) Effects of water and water-mimicking solvents on the lipase-catalyzed esterification in an apolar solvent. Chem. Lett. 1203–1206.
Klibanov, AM (1990) Asymmetric transformations catalyzed by enzymes in organic solvents. Acc. Chem. Res. 23: 114–120.
Mcdaniel DH, Brown HC (1958) An extended table of Hammett substituent constants based on the ionization of substituted benzoic acids. J. Org. Chem. 23: 420–427.
Nakamura K, Takebe Y, Kitayama T, Ohno A (1991) Effect of solvent structure on enantioselectivity of lipase-catalyzed transesterification. Tetrahedron Lett. 32: 4941–4944.
Nakamura K, Kawasaki M, Ohno A (1996) Lipase-catalyzed transesterification of aryl-substituted alkanols in an organic solvent. Bull. Chem. Soc. Jpn. 69: 1079–1085.
Nishio M, Hirota M, Umezawa Y (1998) The CH/π Interaction, Wiley-VCH: New York.
Okamoto T, Ueji S (1999) Drastic enhancement of the enatioselectivity of lipase-catalyzed esterification in organic solvents by the addition of metals ions. Chem. Commun. 10: 939–940.
Oki M, Iwamura H, Onoda T, Iwamura M (1968) Intramolecular interaction between a hydroxyl group and π-electrons-XXII. Tetrahedron 24: 1905–1921.
Parida S, Dordick JS (1991) Substrate structure and solvent hydrophobicity control lipase catalysis and enantioselectivity in organic media. J. Am. Chem. Soc. 113: 2253–2259.
Parida S, Dordick JS (1993) Tailoring lipase specificity by solvent and substrate chemistries. J. Org. Chem. 58: 3238–3244.
Pauling L (1960) Nature of Chemical Bond, 3rd edn. Ithaca, NY: Cornell University Press, pp. 260, 263.
Santaniello E, Ferraboschi P, Grisenti P, Manzocchi A (1992) The biocatalytic approach to the preparation of enantiomerically pure chiral building blocks. Chem. Rev. 92: 1071–1140.
Ueji S, Nakatsu K, Yoshioka H, Kinoshita K (1982) X-ray and IR studies on crystal molecular structure of 4-nitro-2,6-diphenylphenol. Stereochemistry of bifurcated OH...π hydrogen bonds. Tetrahedron Lett. 23: 1173–1176.
Witiak DT, Ho TC-L, Hackney RE (1968) Hypocholesterolemic agents. Compounds related to ethyl α-(4-chlorophenoxy)-α-methylpropionate. J. Med. Chem. 11: 1086–1089.
Yasufuku Y, Ueji S (1995) Effect of temperature on lipase-catalyzed esterification in organic solvent. Biotechnol. Lett. 17: 1311–1316.
Yasufuku Y, Ueji S (1997) High temperature-induced high enantioselectivity of lipase for esterification of 2-phenoxypropionic acids in organic solvent. Bioorg. Chem. 25: 88–99.
Zaks A, Klibanov AM (1984) Enzymatic catalysis in organic media at 100°C. Science 224: 1249–1251.
Zaks A, Klibanov AM (1988) The effect of water on enzyme action in organic media. J. Biol. Chem. 263: 8017–8021.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ueji, Si., Watanabe, K., Koshiba, T. et al. Lipase-catalyzed esterification of 2-(4-substituted phenoxy)propionic acids in organic solvents: substituent effect controlling enantioselectivity toward racemic acids. Biotechnology Letters 21, 865–868 (1999). https://doi.org/10.1023/A:1005522124925
Issue Date:
DOI: https://doi.org/10.1023/A:1005522124925