Abstract
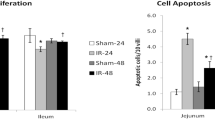
The intestinal mucosa is in a steady state of turnover as the rate of cellular proliferation is balanced by the rate of cell death. Although it is accepted that adaptation after small bowel resection (SBR) results in increased proliferation, its effect on apoptosis is not known. The purpose of this study was to determine the effect of adaptation following SBR on rates of enterocyte apoptosis. Male ICR mice underwent either 50% proximal SBR or sham operation (bowel transection/reanastomosis). After 12 and 24 hours, and 3 and 7 days, rates of proliferation were measured in the ileum as the percentage of crypt cells incorporating bromodeoxyuridine. Apoptosis was quantitated by end labeling of DNA strand breaks and propidium iodide staining of the number of apoptotic bodies per crypt and villus. Significant increases in enterocyte proliferation (30% to 40%) as well as apoptosis (57% to 87%) occurred at all time points following SBR when compared with sham-operated mice. Adaptation following SBR increases both the rate of enterocyte proliferation and the rate of apoptosis. Understanding the pathophysiology of intestinal adaptation and therapeutic interventions designed to augment this important response will require complete characterization of their effects on both proliferation and apoptosis.
Similar content being viewed by others
References
Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intes- tine. I. Columnar cell. AmJ Anat 1974;141:461–479.
Messier B, Leblond CP. Cell proliferation and migration as revealed by radioantography after injection of thymidine-H3 into male rats and mice. AmJ Anat 1960;106:247–285.
Weser E, Tawil T. Epithelial cell loss in remaining intestine after small bowel resection in the rat. Gastroenterology 1976;71:412–415.
Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992;119:493–501.
Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: The importance of apoptosis. J Cell Sci 1994; 107:3569–3577.
Hermiston ML, Gordon JI. In vivo analysis ofcadherin function in the mouse intestinal epithelium: Essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol 1995;129:489–506.
Walker PR, Smith C, Youdale T, Leblanc J, Whitfield JF, Sikorska M. Topoisomerase II-reactive chemotherapeutic drugs induce apoptosis in thymocytes. Cancer Res 1991; 51:1078–1085.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Mont-gomery CA Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992;356:215–221.
Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg 1996;183:441–449.
Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the calorimetric estimation of deoxyribonucleic acid. Biochem J 1956;62:315–323.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275.
Dowling RH, Booth CC. Structural and functional changes following small intestinal resection in the rat. Clin Sci 1967;32:139–149.
Potten CS. What is an apoptotic index measuring? A commentary. Br J Cancer 1996; 74:1743–1748.
Merritt AJ, Potten CS, Watson AJ, Loh DY, Nakayama K, Hickman JA. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci 1995;108:2261–2271.
Potten CS. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev 1992;11:179–195.
Zanotti S, Polzar B, Stephan H, Doll U, Niessing J, Mannherz HG. Localization of deoxyribonuelease I gene transcripts and protein in rat tissues and its correlation with apoptotic cell elimination. Histochem Cell Biol 1995; 103:369–377.
Polzar B, Zanotti S, Stephan H, Ranch F, Peitsch MC, Irmler M, Tschopp J, Mannherz HG. Distribution of deoxyribonu-clease I in rat tissues and its correlation to cellular turnover and apoptosis (programmed cell death). Eur J Cell Biol 1994;64:200–210.
Arai T, Kida Y, Harmon BV, Gobe GC. Comparative alterations in p53 expression and apoptosis in the irradiated rat small and large intestine. Br J Cancer 1996;74:406–412.
Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res 1994;54:614–617.
Korsmeyer SJ. Bcl-2: An antidote to programmed cell death. Cancer Surv 1992; 15:105–118.
Krajewski S, Bodrug S, Krajewska M, Shabaik A, Gascoyne R, Berean K, Reed JC. Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl1 in control of programmed cell death in vivo. Am J Pathol 1995;146:1309–1319.
Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993;74:609–619.
Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol 1994;145:1323–1336.
Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lind-sten T, Turka LA, Mao X, Nunez G, Thompson CB. bct-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993;74:597–608.
Watson AJ. Review article: Manipulation of cell death--The development of novel strategies for the treatment of gastrointestinal disease. Aliment Pharmacol Ther 1995;9:215–226.
Liu Q, Du XX, Schindel DT, Yang ZX, Rescorla FJ, Williams DA, Grosfeld JL. Trophic effects ofinterleukin-11 in rats with experimental short bowel syndrome. J Pediatr Surg 1996;31:1047–1050.
Orazi A, Du X, Yang Z, Kashai M, Williams DA. Interleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab Invest 1996;75:33–42.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a Trustee’s Grant from the Children’s Hospital Research Foundation awarded to Dr. Warner.
Rights and permissions
About this article
Cite this article
Helmrath, M.A., Erwin, C.R., Shin, C.E. et al. Enterocyte apoptosis is increased following small bowel resection. J Gastrointest Surg 2, 44–49 (1998). https://doi.org/10.1016/S1091-255X(98)80102-9
Issue Date:
DOI: https://doi.org/10.1016/S1091-255X(98)80102-9