Abstract
Aim of the study
Notch signaling plays important roles in maintaining intestinal epithelial homeostasis. When Notch signaling is blocked, proliferation ceases and epithelial cells become secretory. The purpose of the present study was to evaluate the role of Notch signaling pathway following intestinal ischemia–reperfusion (IR) injury in a rat model.
Materials and methods
Male Sprague–Dawley rats were randomly divided into four experimental groups: Sham-24 and Sham-48 rats underwent laparotomy and were killed 24 or 48 h later, respectively; IR-24 and IR-48 rats underwent occlusion of SMA and portal vein for 30 min followed by 24 or 48 h of reperfusion, respectively. Enterocyte proliferation and enterocyte apoptosis were determined at killing. Notch-related gene and protein expression were determined using Real Time PCR, Western blotting and immunohistochemistry 48 h followed IR.
Main results
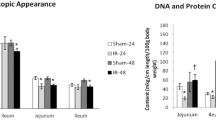
IR-48 rats demonstrated significantly increased rates of cell proliferation and increased cell apoptosis in both jejunum and ileum compared to Sham rats. IR-48 rats exhibited a significant decrease in Notch-1 protein expression (Western blot) that was coincided with a significant decrease in the number of Notch-1 positive cells (immunohistochemistry) in jejunum (35% decrease, p < 0.05) and ileum (twofold decrease, p < 0.05) as well as Hes-1 positive cells in jejunum (28% decrease, p < 0.05) and ileum (31% decrease, p < 0.05) compared to Sham-48 rats.
Conclusions
Forty-eight hours following intestinal IR in rats, accelerated cell turnover was associated by inhibited Notch signaling pathway. Intestinal stem cells differentiation toward secretory progenitors rather than differentiation toward absorptive cells is important at this phase of intestinal recovery.





Similar content being viewed by others
References
Carden DL, Granger DN (2000) Pathophysiology of ischemia-reperfusion injury. J Pathol 190:255–266
Mangino JE, Kotadia B, Mangino MJ (1996) Characterization of hypothermic intestinal ischemia-reperfusion injury in dogs. Effects of glycine. Transplantation 62:173–178
Schoenberg MH, Poch B, Younes M et al (1991) Involvement of neutrophils in postischemic damage to the small intestine. Gut 32:905–912
Yamamoto S, Tanabe M, Wakabayashi G et al (2001) The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia-reperfusion injury of the rat small intestine. J Surg Res 99:134–141
Ikeda H, Suzuki Y, Suzuki M et al (1998) Apoptosis is a major mode of cell death caused by ischemia and ischemia/reperfusion injury to the rat intestinal epithelium. Gut 42:530–537
Noda T, Iwakiri R, Fujimoto K et al (1998) Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J PhysiolGastrointest Liver Physiol 274:G270–G276
Ben-Shahar Y, Pollak Y, Bitterman A et al (2019) Sonic hedgehog signaling controls gut epithelium homeostasis following intestinal ischemia–reperfusion in a rat. PediatrSurg Int 35:255–261
De Santa Barbara P, van den Brink GR, Roberts DJ (2003) Development and differentiation of the intestinal epithelium. Cell Mol Life Sci 60:1322–1323
Kopan R, Ilagan MXG (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233
Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F (2008) Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27 Kip1and p57 Kip2. EMBO Rep 9:377–383
Cheng H, Leblond CP (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141:537–561
Vander Flier LG, Clevers H (2009) Stem cells, self-renewal, and differentiation in the intestinal epithelium. Ann Rev Physiol 71:241–260
Fre S, Huyghe M, Mourikis P, Robine S, Louvard D (2005) Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435:964–968
Schroder N, Gossler A (2002) Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr Patterns 2:247–250
Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY (2001) Requirement of Math 1 for secretory cell lineage commitment in the mouse intestine. Science 294:2155–2158
Ben-Shahar Y, Pollak Y, Bitterman A, Coran AG, Bejar IN, Sukhotnik I (2019) Sonic hedgehog signaling controls gut epithelium homeostasis following intestinal ischemia–reperfusion in a rat. Pediatr Surg Int 35:255–261
Haegebarth A, Clevers H (2009) Wntsignaling, Lgr5, and stem cells in the intestine. Am J Pathol 174:15–21
Van de Wetering M, Sancho E, Verweij C (2002) The β-catenin/TCF-4 complex impoves a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241–250
Guy Sander R, Powel BC (2004) Powell expression of notch receptors and ligands in the adult gut. J HistochemCytochem 52:509–516
Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776
Gridley T (1997) Notch signaling in vertebrate development and disease. Mol Cell Neurosci 9:103–108
Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P (2011) Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 192:767–780
Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F (2011) Dll1- and Dll4-mediated Notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 140:1230–1240
Van Es JH, van Gijn ME, van den Riccio O, Born M, Vooijs M (2005) Notch/gamma-secretase inhibition turns proliferativecells in intestinal crypts and adenomas into goblet cells. Nature 435:959–963
Sukhotnik I, Brod V, Lurie M et al (2009) The effect of 100% oxygen on intestinal preservation and recovery following ischemia-reperfusion injury in rats. Crit Care Med 37:1054–1061
Van Doren M, Bailey AM, Esnayra J, Ede K, Posakony JW (1994) Negative regulation of proneural gene activity: Hairy is a direct transcriptional repressor of achaete. Genes Dev 8:2729–2742
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ben-Shahar, Y., Abassi, Z., Pollak, Y. et al. Accelerated cell turnover 48 h after intestinal ischemia is NOTCH independent. Pediatr Surg Int 35, 1413–1420 (2019). https://doi.org/10.1007/s00383-019-04569-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-019-04569-z