Abstract
Objectives
To investigate the clinical and epidemiological factors associated with severe COVID-19 cases in hospitalized patients across two emirates within the United Arab Emirates (UAE).
Methods
A retrospective observational analytical study analysed data from 738 medical records and conducted 573 in-depth interviews with patients hospitalized across multiple healthcare centers in the UAE, between 29 January 2020 and 14 October 2021. Regression analysis predicted risk factors for COVID-19 severity.
Results
Main risk factors identified were crowding (aOR 1.919; 95%CI 1.144, 3.221), obesity (aOR 2.383; 95%CI 1.332, 4.263), diabetes (aOR 11.14; 95%CI 2.653–46.797), severe dehydration (aOR 3.219; 95%CI 2.161, 4.795), cough or sore throat (aOR 1.607; 95%CI 1.032, 2.502), shortness of breath (aOR 1.921; 95%CI 1.294, 2.853), increased days from symptom onset to admission (aOR 1.055; 95%CI 1.006, 1.105), elevated ANC (aOR 1.263, 95%CI 1.121, 1.424), and AST/SGOT (aOR 1.055, 95% CI 1.016, 1.095). Protective factors included smoking (aOR 0.367; 95%CI 0.182, 0.740), first dose of COVID-19 vaccination (aOR 0.595; 95%CI 0.377, 0.93), higher oxygen saturation (aOR 0.853; 95%CI: 0.801, 0.907) and elevated ALC (aOR 0.540; 95%CI 0.323, 0.905).
Conclusion
Identifying risk factors is crucial for high-risk individuals who may require closer monitoring to improve their outcomes. This can provide guidance for surveillance systems and early detection strategies to mitigate the impact of future outbreaks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
On May 5, 2023, the World Health Organization (WHO) declared that the Coronavirus Disease 2019 (COVID-19) pandemic no longer posed a public health emergency of international concern [1]. However, the disease is still affecting populations worldwide, leading to over 680 million confirmed cases and 6.5 million deaths. As of June 20, 2023, the United Arab Emirates (UAE) had reported 1,067,030 confirmed COVID-19 cases and 2349 deaths [2].
COVID-19 severity and clinical manifestations vary across geographical locations and host factors. Severe patients may exhibit various symptoms and laboratory abnormalities including respiratory system symptoms, musculoskeletal problems, gastrointestinal complaints, mucocutaneous symptoms [3], lymphopenia, thrombocytopenia, abnormal liver function tests, and elevated inflammatory markers [4, 5].
Previous studies have identified predictors of COVID-19 severity including sociodemographic factors such as ethnicity, gender, and residence area [6, 7]; as well as comorbidities like diabetes, hypertension, kidney diseases, nervous system disorders, and obesity [8]. Smoking status has also shown conflicting associations with some studies suggesting it as a potential predictor of adverse COVID-19 outcomes [9], while others associate current smoking to a lower risk of severe COVID-19 [10]. The UAE's diverse population, influenced by variations in ethnicity, genetics, culture, and social practices may significantly impact the spread and severity of COVID-19 [7, 11].
Previous studies in the UAE focused primarily on COVID-19 severity using medical records [8] specific populations such as paediatric patients [12], were confined to a single emirate [8], or a single healthcare facility [13]. This study aims to address these gaps by using two methods of data collection across multiple healthcare centres in more than one emirate. This approach will obtain more precise and reliable data, explore novel relationships, increase the access and diversity to eligible study subjects, and improve generalization of the results.
2 Methods
2.1 Study Design and Setting
A retrospective observational study was conducted in four major hospitals in Sharjah and Dubai, UAE. These hospitals comprised a mix of private, semi-governmental, and governmental institutions offering a wide range of clinical services, and specialized ICU units. The private hospital offered 254 beds, while the semi-governmental hospitals provided 417 and 325 beds, respectively. The governmental hospital had 762 beds. These hospitals were selected for their capacity, range of specialties, and to ensure diverse representation in the study.
2.2 Participants and Sample Size
This study included adults aged 18 years or older who tested positive for COVID-19 using the Real time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) test on nasal and/or pharyngeal swab specimens in laboratories, and who were admitted to one of the four selected hospitals between January 29, 2020, and October 14, 2021. Pregnant women and those unable to provide informed consent were excluded from the study. Each participating hospital provided a list of inpatients who met the inclusion criteria. Participants were then sorted in ascending order based on their hospital ID numbers. We performed a systematic random selection from these lists at predetermined regular intervals where every third person in the list was chosen.
For this study, a minimum of 385 patient records were needed, using the formula Sample Size = [z2 * p (1-p)] / e2, assuming a prevalence of 50%, a margin of error of 5%, and a confidence level of 95%. However, considering a non-response and incomplete response rate of 20%, the target minimum sample size was adjusted to 482 adults.
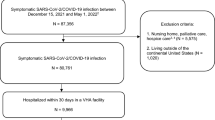
The final achieved sample was 738 for medical records and 573 for the detailed epidemiological telephone interviews. A detailed description of how the final sample size was achieved, as well as a breakdown of participants who were included, did not respond, did not consent, and the number of inaccessible medical records are presented in Fig. 1.
2.3 Data Collection and Study Tools
The study utilized data from both medical records, as well as in-depth interviews with COVID-19 hospitalized patients. The interview guide was initially prepared in English and translated into Arabic and Urdu by the study team. Back-translations were performed to ensure equivalence reliability. Before being sent to five specialists for content validation, 15 adults piloted the interview guide. Following the pilot study, the research team modified the study guide slightly to enhance its efficacy and validity. To ensure inter-rater reliability all interviewers and data collectors underwent the same training sessions.
The process of data collection was performed as follows. First, patients or their next of kin were invited to participate through telephone calls. Those who expressed willingness and consent were offered a convenient interview date and time. Those who declined to participate in the study were recorded as non-response. Next, the participants' medical data were retrieved. Finally, the interviews were conducted on the agreed-upon day and time.
The structured interview which collected information on sociodemographic characteristics, behavioral risk factors, and vaccination status took approximately ten minutes to complete.
Medical data were extracted from each patient’s electronic health records using a standardized data collection form. This form was a modified version of the WHO/International Severe Acute Respiratory and Emerging Infection Consortium acute respiratory infections (ISARIC) case record form. It included the following sections: severity on admission, past medical history, BMI categories (underweight < 18.5 kg/m2, normal 18.5–24.9 kg/m2, overweight 25.0–29.9 kg/m2, and obese > 29.9 kg/m2) [14], radiological testing, symptoms, clinical measures, and laboratory test results. Any missing or uncertain records were clarified through direct communication with healthcare providers or participants.
COVID-19 severity on admission was categorized into four categories following the National Health Commission of China guidelines that were available when we designed the study. The classification was defined as follows: (i) Mild: presented with mild symptoms without radiographic features; (ii) Moderate: presented with fever, respiratory symptoms, and radiographic features; (iii) Severe: met one of the three criteria: (a) dyspnea, respiration rate (RR) greater than 30 times/min, (b) oxygen saturation less than 93% in ambient air, and (c) PaO2/FiO2 less than 300 mm Hg; and (iv) Critical: met one of the following criteria: (a) respiratory failure, (b) septic shock, or (c) multiple organ failure [15]. To simplify regression analysis, COVID-19 severity was further grouped into two categories: non-severe (including mild) and severe (including, moderate, severe, and critical).
2.4 Data Analysis
Data were analysed using SPSS version 28 [16]. Descriptive data were analysed using counts and percentages, and normality was tested using the Kolmogorov–Smirnov test. Associations between categorical variables were explored using Chi-square and Fisher’s exact tests, while two group comparisons involving continuous variables were conducted using independent samples t-test and Mann–Whitney U tests. Multivariate binary logistic regression analysis was conducted to predict the odds of severe COVID-19. Independent variables included in the models were selected based on statistical significance, while variables with small-number observations were excluded. Four models were developed: the first focused on sociodemographic characteristics, smoking history and medical history; the second examined symptoms and clinical measures; the third analysed laboratory results; and the final model included all study variables.
3 Results
In total, clinical data from 738 patient medical records were extracted. Out of these, detailed epidemiological telephone interviews were conducted with 573 patients. The larger number of nonresponses in the interviews was attributed to refusal to consent to interviews, withdrawals of the interview consent on the day of the interview, or failure to answer the call during the scheduled appointments. However, the incomplete responses in interviews were due to patients either not completing the entire interviews or refusing to answer specific questions. Additionally, the medical records had gaps due to variations in test performance and information recording among different hospitals.
3.1 Sociodemographic Characteristics and Smoking History of Study Participants
Approximately half of the hospitalized COVID-19 patients were Arab (56.7%), employed (56.9%). and aged 30 to 60 years (61.5%). Most were married (79.1%) and had no family history of consanguinity (78.3%). While 76.5% identified themselves as non-smokers, 62.1% reported that no one in their family smoked (Table 1).
3.2 Past Medical History of Patients
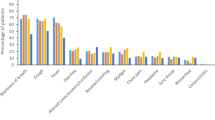
Hypertension (37.9%) and diabetes (37.5%) were the most common comorbidities among participants (Fig. 2). The majority were overweight or obese (75.1%). ACE inhibitors (8.0%) were the most frequently prescribed pre-existing medications, and 38.3% of patients had received the initial dose of the COVID-19 vaccine (Table 1).
3.3 COVID-19 Severity Categories and Symptoms on Admission
Around half of the hospitalized patients had a moderate form of COVID-19 (41.3%) on admission while 29% were classified as mild, 23.8% as severe and only 5% as critical. The most reported symptoms on admission were fever (75.9%), and cough or sore throat (74.7%) (Fig. 3). Lung abnormalities were diagnosed in 90.1% via chest X-ray and in 57.7% via CT scan (Table 1).
3.4 Clinical Measures on Admission
Patients had a median duration of 4 days (IQR 5 days) from symptom onset to hospitalization. Most clinical measurements were within the normal range, except for a slightly elevated median systolic blood pressure at (128 mmHg) (IQR 25) and median body temperature of (37.4 °C) (IQR 1.4 °C) (Table 2).
3.5 Laboratory Results
Upon admission, coagulation testing profiles indicated prolonged prothrombin time and elevated d-dimer levels with median values of 12.3 s (IQR 2.3 s) and 0.72 mg/l (IQR 0.670 mg/L) respectively. Elevated median values of inflammatory markers were also observed, ESR at 53 mm/1 h (IQR 48 mm/1 h), LDH at 367 U/L (IQR 235 U/L), ferritin at 368 ng/mL (IQR-553), procalcitonin 0.14 ng/mL (IQR 0.26 ng/mL), and CRP 74 mg/L (IQR 104.5 mg/L). Other laboratory results, including renal function, liver function, electrolytes, indicators of cardiac injury, and complete blood count were within normal limits (Table 2).
3.6 Bivariate Analysis
Men had a higher percentage of severe cases (74.1%) compared to women (65.0%). Divorced and widowed patients (75.8%) showed higher severity rates compared to married (68.0%) and single (52.7%) patients. Patients aged 65 years or older (74.2%) had a higher proportion of severe cases compared to those aged 30 to 59 years (72.1%) and 18 to 29 years (41.9%). Patients residing in other emirates (77.8%), or Sharjah/Ajman (74.4%) had higher severity rates compared to residents of Dubai (54.0%). Patients with a monthly income less than 15,000 AED (72.0%) had a higher proportion of severe COVID-19 than those with incomes ranging from 15,000 to 30,000 AED (58.4%) and more than 30,000 AED (69.9%). Living in residences with three or more people sharing one room (73.7%) was associated with higher severity rates compared to living in less crowded homes (65.1%). Non-smokers and former smokers (68.9%) had higher frequencies of moderate/severe cases compared to current smokers (59.8%) (Table 3).
A higher percentage of patients who had severe COVID-19 at admission were patients who were obese, had hypertension, diabetes, unknown immunodeficiencies, had not received the BCG vaccine, and had not received the first dose or second dose of the COVID-19 vaccine. A higher percentage of patients with fever (76.3%); cough or sore throat (74.7%); shortness of breath (82.5%); and severe dehydration (84.9%) were significantly associated with severe COVID-19 cases. In contrast, vomiting or nausea (57.7%); abdominal pain (58.0%); runny nose (51.9%); and smell and taste distortion (30.8%) were significantly associated with lower proportions of severe COVID-19 cases (Table 3).
Severe COVID-19 patients had significantly longer median durations from symptom onset to hospital admission (4 days), and higher medians of temperature (37.5 °C), heart rate (93 beats/min) and respiratory rate (20 breaths/min). In contrast, median oxygen saturation SpO2 (%) was significantly lower in severe COVID-19 patients compared to non-severe COVID-19 patients (95) (Table 4).
Notable differences in laboratory results were observed between severe and non-severe cases. Severe cases exhibited higher medians for white blood count (WBC) (6.8 × 10^9/L), absolute neutrophil count (ANC) (5.10 × 10^3/ul), and lower median absolute lymphocyte (ALC) (0.9 × 10^3/ µL). Furthermore, electrolyte and renal function markers in the severe group demonstrated differences, with a lower median sodium concentration (135 mEq/L) and a higher median creatinine level (87.5 µmol/L). Severe COVID-19 patients exhibited significantly higher medians of total bilirubin (9.3umol/l); alanine transaminase (ALT/SGPT) (37.5 U/L); and aspartate transaminase (AST/SGOT) (41 U/L) compared to non-severe patients. On the other hand, the median albumin level was lower in severe COVID-19 patients (31 g/l). Inflammatory markers such as erythrocyte sedimentation rate (ESR) (59.8 mm/1 h), lactate dehydrogenase (LDH) (389 U/L), procalcitonin (0.15 ng/mL), and C-reactive protein (CRP) (83 mg/l) were significantly higher in the severe COVID-19 group compared to the non-severe group (Table 4).
3.7 Multiple Logistic Regression
Table 5 presents results of the multivariable logistic regression analysis. Model 4, which included all significant study variables, provided the best fit to the data with a Pseudo R2 value of 0.738 based on McFadden estimates compared to 0.143 for Model 1 (sociodemographic characteristics, smoking history, and past medical history), 0.334 for Model 2 (symptoms, and clinical measures), and 0.156 for Model 3 (laboratory results).
In Model 1, predictors of worse COVID-19 severity were overweight (aOR 1.371; 95% CI: 0.778, 2.416), obesity (aOR 2.383; 95% CI 1.332, 4.263), area of residence (aOR 2.120; 95% CI 1.332, 4.263), and crowding (aOR 1.919; 95% CI 1.144, 3.221). However, protective factors of worse COVID-19 were smoking status (aOR 0.367; 95% CI 0.182, 0.740) and receiving the first dose of COVID-19 vaccination (aOR 0.595; 95%CI 0.377, 0.939).
Model 2 showed that severe dehydration (aOR 3.219; 95%CI 2.161, 4.795), cough or sore throat (aOR 1.607; 95%CI 1.032, 2.502), shortness of breath (aOR 1.921; 95%CI 1.294, 2.853) and the number of days from symptom onset to admission were associated with increased likelihood of worse COVID-19 (aOR 1.055; 95%CI 1.006, 1.105). On the other hand, for every one unit increase in oxygen saturation, the risk of worse COVID-19 severity decreased by 14.7% (aOR 0.853; 95%CI 0.801, 0.907).
In model 3, ANC and bilirubin levels were positively associated with COVID-19 severity. For every one-unit increase in ANC and bilirubin, the likelihood of worse COVID-19 severity increased by 26.3% (aOR 1.263; 95%CI 1.121, 1.424) and 3.6% (aOR 1.036; 95%CI 1.004, 1.069), respectively. On the other hand, for every one-unit increase in ALC, the odds of worse COVID-19 severity decreased by 46.0% (aOR 0.540; 95%CI 0.323, 0.905).
Model 4 revealed that diabetic patients were approximately 11 times more likely to have severe COVID-19 on admission (aOR 11.14; 95% CI 2.653–46, 797). Additionally, an increase in AST/SGOT was associated with a higher likelihood of severe COVID-19 (aOR 1.055, 95% CI 1.016, 1.095).
4 Discussion
While the WHO declares the end of the COVID pandemic, there remains a critical need to augment the body of evidence for future pandemic management strategies and emphasize the significance of ongoing comprehensive studies. This study provides insights into epidemiological characteristics, clinical aspects, laboratory features, and risk factors of COVID-19 patients hospitalized in the UAE.
Most patients in our study were classified as moderate to severe COVID-19 cases, with only about a third experiencing mild symptoms. This is consistent with a previous study in Abu Dhabi, UAE [17]. The lower percentage of mild hospitalized patients suggests that those with mild symptoms tend to manage their illness through self-care while hospitalization becomes necessary when symptoms worsen. Additionally, the relatively low occurrence of critical cases suggests it would be appropriate to group moderate, severe, and critical cases together as a single category, distinguishing them from patients’ mild symptoms.
Interestingly, our findings revealed that overcrowding was a risk factor for COVID-19 severity. This observation is consistent with a previous study from Canada that linked both the spread and severity of COVID-19 to crowded conditions [18]. Despite the UAE’s reputation as a financial centre with modern infrastructure, the country faces unique challenges due to its substantial population of migrants, including construction laborers, and domestic workers living in shared accommodations. Such living arrangements create an environment conducive to the rapid transmission of infectious diseases [7]. Implementing regulations that specify minimum space per person and ensure adequate ventilation in shared accommodations can play a crucial role in preventing infectious disease transmission and safeguarding the well-being of individuals.
Another interesting finding in our study was the potential protective effect of smoking against severe COVID-19. Traditionally, tobacco products have been associated with negative effects on lung function in individuals with infectious diseases [9]. However, in the case of COVID-19, studies have reported a lower risk of severe COVID-19 outcomes among smokers [10]. One possible mechanism proposed to explain this effect involves nicotine’s potential to reduce the release of pro-inflammatory cytokine [19]. Another probable explanation for the protective effect of smoking against severe COVID-19 in our study population could be related to selection factors. It is possible that smokers in our study were more hospitalized than non-smokers, even in cases when their symptoms were mild, creating an impression that smoking was protective. Nevertheless, the association with COVID-19 is intricate and warrants further investigation.
Consistent with previous findings, our study demonstrated that receiving the first dose of a COVID-19 vaccine provided protection against severe COVID-19. The initial vaccine dose triggers the immune system to produce antibodies and memory cells capable of combating the virus. [20]. While the development of the immune response may vary among individuals and takes time, most COVID-19 vaccines have proven highly effective in reducing hospitalization rates, disease severity, and mortality with just a single dose [21]. Even though our study included participants before the availability of COVID-19 vaccination, the protective impact of the UAE’s nationwide vaccination program was evident. The UAE achieved nationwide immunity by providing free COVID-19 vaccines to all residents and citizens, becoming the first nation to successfully vaccinate 100% of eligible population groups [22].
Our study also found that diabetes and obesity were associated with worse COVID-19 outcomes consistent with previous research [8]. Given the high prevalence of diabetes and obesity in the UAE, understanding the complicated and multidirectional relationship between these conditions and COVID-19 is crucial [23]. Both conditions are recognized as part of the same interconnected pathophysiological mechanism that influences COVID-19 outcomes. Obesity and diabetes can lead to chronic inflammation, compromised immune responses, and alterations in pulmonary function, all contributing to more severe disease outcomes [8, 24].
Regarding symptoms, our results revealed a significant association between COVID-19 severity and the duration of symptoms from onset to hospital admission, aligning with a European study [25]. This alignment suggests that the longer the duration of symptoms before hospitalization, the higher the likelihood of experiencing severe COVID-19 outcomes. Among our severe patients, the most common symptoms included fever, cough, shortness of breath, severe dehydration, and low oxygen saturation levels, indicating an association with acute respiratory distress syndrome (ARDS). ARDS is characterized by rapid breathing, shortness of breath, and reduced oxygen saturation. Dehydration in severe COVID-19 patients can result from viral infection of the gastrointestinal tract or reduced fluid intake due to loss of appetite, nausea, vomiting, or difficulty swallowing [26].
Severe COVID-19 patients in our study had elevated ANC levels and decreased ALC levels. These findings align with a previous meta-analysis, which demonstrated that elevated neutrophil count and decreased lymphocyte count at admission can predict COVID-19 severity [27]. It’s important to note that hospitalized patients may display abnormal laboratory results due to various factors such as systemic inflammation response syndrome, drug-induced liver injury, or multiorgan failure. Therefore, relying solely on these markers as a single biomarker predictor should be approached with caution [6]. One plausible explanation for these findings is that lymphocytes play a key role in regulation of the inflammatory response, and their loss due to persistent infection may lead to immune system suppression and the nonresolution of inflammation. Consequently, the medulla in the brain may produce more neutrophils to combat the invading pathogen [28].
The present study also revealed elevated levels of AST and total bilirubin in severe COVID-19 patients, indicating altercations in liver biochemistry. This association between severe COVID-19 infection and liver involvement has been reported in a systematic literature review conducted by Afra et al. [29]. AST levels typically indicate hepatocyte (liver cell) injury, while elevated bilirubin levels suggest bile duct damage. These markers serve as indicators for assessing the severity of liver involvement in COVID-19 patients and guiding appropriate management strategies. Several factors contribute to the progression of liver injury in COVID-19, including direct viral invasion of liver cells, cytokine storms that trigger liver inflammation and damage, and ischemia resulting from impaired blood flow to the liver [29, 30].
5 Strengths and Limitations
To the best of our knowledge, this study represents the first comprehensive examination of clinical and epidemiological characteristics of COVID-19 hospitalized patients in the UAE, encompassing multiple centres and spanning various phases of the pandemic. The study provides insights on potential risk factors associated with the severity of COVID-19, which can aid decision makers and inform population level public health strategies. Although prior infections and vaccinations have significantly reduced the overall risk of severe outcomes compared to the early stages of the pandemic, the risk factors identified in this study are likely to continue influencing relative risks in the future.
However, our study does have limitations. The clinical homogeneity of our study participants was compromised due to the 21-month period during which the clinical landscape of COVID-19 naturally evolved. The emergence of different variants of the virus across waves of the pandemic may have contributed to evolving clinical presentations of the disease over time. We were unable to consider the strains in our analysis since the data in the medical records of the four participating hospitals lacked this information. Furthermore, our study had low response rate, which was mostly attributed to the fact that many of the participants were non reachable expatriates who had left the country or changed their phone numbers. In addition, incomplete laboratory results exist as not all patients underwent the same battery of tests across participating hospitals. The absence of uniform data across all cases may affect the comprehensiveness of our analysis, potentially missing critical information that could further elucidate COVID-19 characteristics and risk factors. Moreover, our study may be susceptible to survival bias as it excluded individuals who passed away during the follow-up period. This exclusion may skew our findings towards less severe cases and fail to capture valuable insights into the full spectrum of COVID-19 severity.
However, while we acknowledge the constraints and potential biases inherent in our research, these limitations do not diminish the importance and knowledge gained from this study. Our findings offer insights that can significantly benefit our understanding and management of COVID-19.
6 Conclusion
Our findings highlight the association between crowding, obesity, diabetes, severe dehydration, cough or sore throat, shortness of breath, increased days from symptoms onset to admission, elevated ANC levels, and elevated AST/SGOT levels with an increased risk of severe COVID-19 symptoms. This underscores the importance of rigorous screening protocols in healthcare facilities, enabling early identification and intervention for individuals with these risk factors. Incorporating risk factors into surveillance protocols will help healthcare systems identify high risk individuals, allocate resources efficiently and respond rapidly to outbreaks ultimately improving public health outcomes.
Data Availability
The data that support the findings of this study are not openly available due to reasons of confidentiality and are available from the corresponding author upon reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease of 2019
- UAE:
-
United Arab Emirates
- aOR:
-
Adjusted odds ratio
- CI:
-
Confidence intervals
- AST/SGOT:
-
Aspartate aminotransferase/serum glutamic-oxaloacetic transaminase
- ALC:
-
Absolute lymphocyte count
- ANC:
-
Absolute neutrophile count
- WHO:
-
World Health Organization
- RT-PCR:
-
Real time Reverse Transcriptase Polymerase Chain Reaction
- ISARIC:
-
International Severe Acute Respiratory and Emerging Infection Consortium
- BMI:
-
Body Mass Index
- PaO2:
-
Partial pressure of arterial oxygen
- FiO2:
-
Fraction of inspired oxygen
- ACE:
-
Angiotensin-converting enzyme inhibitors
- ARBS:
-
Angiotensin II receptor blockers
- NSAID:
-
Non-steroidal anti-inflammatory drug
- BCG:
-
Bacillus calmette-guérin
- GGT:
-
Gamma-glutamyl transferase
- aPTT:
-
Activated partial thromboplastin time
- ESR:
-
Erythrocyte sedimentation rate
- CK:
-
Creatinine kinase
- IgG:
-
Immunoglobin G
- LDH:
-
Lactate dehydrogenase
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- INR:
-
International normalized ratio
- CBC:
-
Complete blood count
- WBC:
-
White blood count
- Hb:
-
Hemoglobin
- PT:
-
Prothrombin time
- GGT:
-
Gamma-glutamyl transferase
- LDH:
-
Lactate dehydrogenase
- CRP:
-
C-reactive protein
- IQR:
-
Interquartile range
- BP:
-
Blood pressure
References
WHO_a. World Health Organization (WHO). Statement on the fifteenth meeting of the IHR Emergency Committee on the COVID-19 pandemic. 2023 Accessed on 31/03/2023]; Available from: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations- (2005)-emergency-committee-regarding-the-coronavirus-disease- (covid-19)-pandemic.
Wordlometers. COVID-19 Coronavirus Pandemic. Reported Cases and Deaths by Country or Territory. 2023 Accessed on 20–06–2023]; Available from: https://www.worldometers.info/coronavirus/
Docherty AB, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369: m1985.
Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506.
Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335–1335.
Tan JY, et al. A comparative study on the clinical features of COVID-19 with non-SARS-CoV-2 respiratory viral infections. J Med Virol. 2021;93(3):1548–55.
GMI. United Arab Emirates Ppopulation Statistics. 2023 Accessed on 20–06–2023]; Available from: https://www.globalmediainsight.com/blog/uae-population-statistics/#:~:text=Majority%20of%20the%20UAE%20population,for%20Dubai%20is%2033.5%20years.
Al Bastaki NA, et al. An evaluation of non-communicable diseases and risk factors associated with COVID-19 disease severity in Dubai, United Arab Emirates: an observational retrospective study. Int J Environ Res Public Health. 2022;19(21):14381.
He Y, et al. Association between smoking and COVID-19 severity: a multicentre retrospective observational study. Medicine (Baltimore). 2022;101(29): e29438.
Simons D, et al. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7). Addiction. 2021;116(6):1319–68.
Irizar P, et al. Ethnic inequalities in COVID-19 infection, hospitalisation, intensive care admission, and death: a global systematic review and meta-analysis of over 200 million study participants. EClinicalMedicine. 2023;57: 101877.
Al Mansoori L, et al. Epidemiological characteristics of children with coronavirus at a joint commission-accredited hospital in the United Arab Emirates. J Family Med Prim Care. 2021;10(6):2348–52.
Hachim IY, et al. The inflammatory biomarkers profile of hospitalized patients with COVID-19 and its association with patient’s outcome: a single centered study. PLoS ONE. 2021;16(12): e0260537.
WHO_b, A Healthy Lifestyle-WHO Recommendations. 2010. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations#:~:text=To%20ensure%20a%20healthy%20lifestyle,promote%20and%20support%20healthy%20lifestyles. Aaccessed on 20–06–2023.
Wu Y, et al. Evolution and major changes of the diagnosis and treatment protocol for COVID-19 patients in China 2020–2023. Health Care Sci. 2023;2:135–152. https://doi.org/10.1002/hcs2.45
IBM Crop. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp.
Hosani FA, et al. Epidemiology of asymptomatic and symptomatic Coronavirus Disease 2019 confirmed cases in the Emirate of Abu Dhabi, United Arab Emirates: observational study. Medicine (Baltimore). 2021;100(12): e25219.
Brown KA, et al. Association between nursing home crowding and COVID-19 infection and mortality in Ontario. Canada JAMA Intern Med. 2021;181(2):229–36.
Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19(6):493–9.
Barnes E, et al. SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease. Nat Med. 2023;29(7):1760–74.
Rotshild V, et al. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11(1):22777.
UAE portal, 2022. Vaccines against COVID-19 in the UAE. Accessed on 22–06–2023. ]; Available from: https://u.ae/en/information-and-services/justice-safety-and-the-law/handling-the-covid-19-outbreak/vaccines-against-covid-19-in-the-uae.
Al Sabbah H, et al. Nutrition situation analysis in the UAE: a review study. Nutrients. 2023;15(2):363.
Kruglikov IL, Shah M, Scherer PE. Obesity and diabetes as comorbidities for COVID-19: Underlying mechanisms and the role of viral-bacterial interactions. Elife. 2020. https://doi.org/10.7554/eLife.61330.
Palmieri L, et al. Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. J Gerontol A Biol Sci Med Sci. 2020;75(9):1796–800.
Pan L, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional. Multicenter Study Am J Gastroenterol. 2020;115(5):766–73.
Henry B, et al. Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: a meta-analysis. Acta Biomed. 2020;91(3): e2020008.
Liu X, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in patients with sepsis: a prospective observational study. Mediators Inflamm. 2016;2016:8191254.
Shokri Afra H, et al. Positive association between severity of COVID-19 infection and liver damage: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2020;13(4):292–304.
Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13(3):267–76.
Funding
This work was supported by grants from, Clinical Epidemiology Research group operational grant (Grant code:150389) and COVID-19 research grant (CoV19-0301), University of Sharjah, UAE.
Author information
Authors and Affiliations
Contributions
BS, HA, MA, AH, MSA, QH, RH: conceived and designed the study. BM, RB, HE, MS and GM: made substantial contributions in reviewing the design of the study and acquiring the data. BS, AS, RZ, HIA, ZA, DW, MA, KA, MBA and LS coordinated sample collection and oversaw data collection. NA, RA and AS: analysed the data and BS, RZ, HA, MA, AH, RB, BM, HE, MS and GM: interpreted the data. NA and RA: conducted the literature review and drafted the manuscript. NA, RA, AS, RZ, HA, MA, MSA, QH, RH and BS: contributed by revising the manuscript critically for important intellectual content. MA, KA, MBA, LS, DW, ZA and HIA: critically reviewed the manuscript. All authors contributed to final approval of the version to be submitted.
Corresponding author
Ethics declarations
Conflict of Interest
The authors state that they have no known competing financial interests or personal relationships that might have appeared to affect the work reported in this paper.
Ethical Approval
Ethical approval for the study was obtained from the Research Ethics Committees at Ministry of Health and Prevention (MOHAP) (MOHAP/DXB-REC/SSS/No.124/2020), University of Sharjah Hospital (UHS-HERC-098–10042022) and Dubai Health Authority (DSREC-09/2021_14).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Bluwi, N., Agha, R., Shukla, A. et al. Epidemiological and Clinical Characteristics Associated with COVID-19 Severity Among Hospitalized Patients in the United Arab Emirates: A Retrospective Multicentre Study. J Epidemiol Glob Health 14, 349–362 (2024). https://doi.org/10.1007/s44197-024-00206-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44197-024-00206-8