Abstract
All-cause COVID-19 hospitalization ≤ 30 days of infection is a common outcome for severe illness in observational/surveillance studies. Milder COVID-19 disease and COVID-19-specific measurements calls for an evaluation of this endpoint. This was a descriptive, retrospective cohort study of adults ≥ 18 who were established in primary care at Veteran Health Administration (VHA) facilities. The outcome was hospitalization within 30 days of a laboratory-confirmed, symptomatic SARS-CoV-2 infection. Between December 15, 2021 and May 1, 2022, a simple random sample of all VA facilities, excluding Puerto Rico or Philippines, was drawn to identify these hospitalized cases and determine whether hospitalization was due to COVID-19-specific causes. A chart review was conducted to record the inpatient clinical team’s diagnosis and whether the inpatient team classified the diagnosis as COVID-19 related or not. These data were used to classify hospitalizations as either due to COVID-19-specific causes (direct manifestations of SARS-CoV-2 infection) or non-COVID-19-specific hospitalizations (incidental SARS-CoV-2 infection), A simple random sample of 9966 (12.3%) all-cause hospitalizations (95% CI: 12.1%, 12.5%) was used to select 300 representative patients. Of these, 226/300 (75.3%) were determined to be COVID-19-specific. COVID-19 pneumonia was most common (147/226, 65.0%). The highest proportion of COVID-19-specific hospitalizations occurred among unvaccinated (85.0%), followed by vaccinated but not boosted (73.7%) and boosted (59.4%) (p < 0.001). The proportion of non-COVID-19-specific hospitalizations was higher in the later period (15–30 days: 55.0%) than the early (0–15 days: 22.5%) (p = 0.003). This study supports the outcome of COVID-19-specific hospitalization instead of all-cause hospitalization in observational studies. The earlier outcome period (0–15 days) was less susceptible to potential measurement bias.
Similar content being viewed by others
Introduction
Observational, surveillance studies rely on readily available data from electronic health records, insurance databases, or other sources to assess the effectiveness of COVID-19 vaccines or therapeutics through a target trial emulation approach1,2,3,4,5. This approach uses all-cause hospitalization after symptomatic COVID-19 as a proxy for COVID-19-related hospitalization with the intent to measure severe illness. In contrast, clinical trials of COVID-19 vaccines and therapeutics have the intensive resources to directly measure COVID-19-related hospitalization6,6,8. The U.S. Veterans Health Administration (VHA) has access to detailed, patient-level data so that chart reviews can be conducted, offering the ability to evaluate all-cause COVID-19 hospitalization for mismeasurement biases.
In an era of vaccines, increasing natural immunity, and viral evolution, it is important to re-evaluate all-cause COVID-19 hospitalization as a useful surrogate outcome in observational studies because COVID-19 disease has been associated with less severe illness and complications, including pneumonia, acute myocardial infarction, and ischemic stroke after infection9. Without the late occurrence of cytokine storms, progression to severe illness typically occurs over a shorter period, suggesting that measurement of all-cause COVID-19 hospitalization over a 30-day period may be more likely to detect hospitalizations unrelated than related to COVID-19. At the same time, COVID-19 remains a relatively common occurrence10 and infected, symptomatic individuals can be hospitalized for many reasons that may be unrelated to COVID-19 disease (incidental SARS-CoV-2 infections)10.
During a period with a predominance of Omicron SARS-CoV-2 variants, this study drew a random sample of all-cause COVID-19 hospitalizations among a national cohort of U.S. Veterans. Reasons for hospitalization documented by the inpatient care team in medical charts were abstracted and used to determine whether the hospitalization was due to a COVID-19-specific cause.
Methods
Ethics statement
The institutional review board of the University of California, San Francisco, approved this study and waived requirement for patient consent, due to the retrospective nature of the study. All methods were performed in accordance with the relevant guidelines and regulations. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study design, data sources, and participants
We conducted a retrospective cohort study using chart review to describe the proportion of all-cause hospitalizations due to COVID-19-specific or non-COVID-19-specific causes among hospitalized patients with laboratory-confirmed, symptomatic SARS-CoV-2 infection. The cohort included adults aged 18 or older who were established at the U.S. VHA facilities. Exclusion criteria included (1) use of nursing home or hospice care services within the 2 years prior to a COVID-19 diagnosis, and (2) hospitalization in Puerto Rico or the Philippines. We used VA Corporate Data Warehouse (CDW)11 and COVID-19 shared data resource12 to construct the cohort and identify patients who had laboratory-confirmed, symptomatic SARS-CoV-2 infection and were hospitalized within 30 days of infection. COVID-19 shared data resource was established to document symptomatic infections and hospitalizations occurring outside of VA facilities and integrate these data back into the VA health records. A random sample of hospitalizations was drawn for chart review between December 15, 2021 and May 1, 2022. Thirty percent of these charts were reviewed in duplicate.
Measurements
When reviewing charts, study staff recorded the inpatient clinical team’s diagnosis and whether the inpatient team classified the diagnosis as COVID-19 related or not. Diagnoses related to COVID-19 were defined as COVID-19-specific (direct manifestations of SARS-CoV-2 infection), and those unrelated to COVID-19 were defined as non-COVID-19-specific causes (incidental SARS-CoV-2 infection).
Any hospitalization with unclear labeling and/or disagreement amongst the chart abstractors about the diagnosis being related to COVID-19 was flagged for review by three clinicians (JDK, SK, DMB) and consensus was reached via discussion. An example of unclear labeling in a person with symptomatic SARS-CoV-2 infection was pneumonia, either due to bacterial and viral pathogen, so unless these were witnessed aspiration events, the pneumonia was classified as COVID-19-related since bacterial and viral infections were radiologically indistinguishable. The study team did not overrule any diagnoses or etiological statements made by the clinical team.
Covariates used to describe the cohort were extracted from the VA Corporate Data Warehouse. Those with high-risk comorbid and immunocompromising conditions were classified using U.S. Centers for Disease Prevention and Control definitions10,13.
Statistical analysis
The cumulative incidence of all-cause hospitalization was described in the overall cohort and sub-groups stratified by vaccination status (unvaccinated [no vaccine dose], vaccinated but not boosted [received primary series], boosted [received all recommended doses]) and time since infection (0–15 days, 16–30 days). In the random sample, reasons for hospitalization as determined by the abstraction team’s chart review were categorized as COVID-19-specific causes, and non-COVID-19-specific causes. Descriptive statistics with Chi-squared tests were used to compare the proportion of all-cause and cause-specific hospitalizations among those related and not related to COVID-19, stratified by vaccination status and by time since COVID-19 infection. A 2-tailed p < 0.05 was considered significant. Analyses were conducted in R version 1.2.5019.
Results
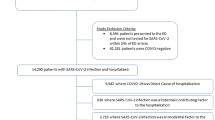
Among 6,634,897 patients receiving care at VHA facilities, 80,761 had laboratory-confirmed, symptomatic SARS-CoV-2 infection (121.7 events per 10,000 persons; 95% CI: 120.9, 122.6). Among these infected patients, there were 9966 (12.3%) all-cause hospitalizations (95% CI: 12.1%, 12.5%). A simple random sample of this hospitalized population was used to select 300 patients who were representative of the national cohort (Fig. 1). Among these 300 hospitalized patients, 196 (65.3%) were aged 65 or older and 17 (5.7%) were female; 198 (66.0%) had high-risk comorbid conditions and 67 (22.3%) had an immunocompromising condition. See Table 1 for additional characteristics.
Among all-cause hospitalizations, 226/300 (75.3%) were determined to be COVID-19-specific. The most common causes of the 226 COVID-19-specific hospitalizations were as follows: COVID-19 pneumonia, 147 (65.0%); cardiovascular events (e.g., myocarditis, new onset atrial fibrillation), 23 (10.2%); weakness/falls, 12 (5.3%); COPD/asthma exacerbation, 8 (3.5%); neurocognitive disorders (e.g., encephalopathy), 8 (3.5%); and gastrointestinal (GI) illness (e.g., diarrhea), 10 (4.0%). The remaining hospitalizations (74/300, 24.7%) were non-COVID-19-specific. The most common causes of the 74 non-COVID-19-specific hospitalizations were as follows: GI illness (e.g., recurrent GI bleeding, abdominal pain from constipation), 17 (23.0%); mental illness or substance abuse (e.g., alcohol withdrawal), 16 (21.6%); non-COVID infectious disease (e.g., urinary tract infection), 15 (20.3%); cardiovascular events (e.g., hypertensive emergency off blood pressure medications), 6 (8.1%); and pain syndrome, 6 (8.1%). See Table 2 for other diagnoses classified as COVID-specific or non-COVID-19-specific hospitalizations. See Supplement for descriptions of these diagnoses during the hospitalization and whether the case was classified as COVID-19-specific or non-COVID-19-specific.
The proportion of COVID-19-specific hospitalizations among all-cause hospitalizations varied by vaccination status. The highest proportion of COVID-19-specific hospitalizations occurred among the unvaccinated group (108/127, 85.0%), followed by the vaccinated but not boosted group (73/99, 73.7%) and boosted group (41/69, 59.4%) (p < 0.001). Stratified by time since infection (0–15 days, 15–30 days), the majority of all-cause hospitalizations (280, 93.3%) occurred within the first 15 days of infection. The proportion of non-COVID-19-specific hospitalizations was higher in the later period (15–30 days: 11/20, 55.0%) than the early period (0–15 days: 63/280, 22.5%) (p = 0.003) (Table 2).
Discussion
In this cohort of patients hospitalized at VHA facilities 30 days after symptomatic SARS-CoV-2 infection, we evaluated the extent to which all-cause hospitalization within 30 days of symptomatic SARS-CoV-2 infection remains a useful outcome for severe COVID-19 illness in observational, surveillance studies. Consistent with the literature14,15, about one-quarter of all-cause hospitalizations were non-COVID-19-specific (incidental SARS-CoV-2 infection). A higher proportion of non-COVID-19-specific hospitalizations occurred among those who were boosted than unvaccinated and among those hospitalized 16–30 days instead of 0–15 days after infection. As the U.S. population has become highly immunized to COVID-19 either through vaccination or infection and as the clinical spectrum of illness has moderated9, continued use of all-cause hospitalization within 30 days of symptomatic SARS-CoV-2 infection as a study outcome should be approached with caution because of its trade-off between sensitivity and specificity.
Hospitalization within 15 days after symptomatic SARS-CoV-2 infection was a more specific measurement of COVID-19 hospitalization than within 30 days and may be less prone to measurement bias. Over time, the likelihood that SARS-CoV-2 infection results in a hospitalization due to COVID-19-specific causes decreases; thus we expect to observe more incidental SARS-CoV-2 infections during this later period (16–30 days). In addition, summary measures of COVID-19-specific hospitalization have yet to be validated. This study found hospitalization due to COVID-19 pneumonia accounted for most COVID-19-specific causes and can be identified with an ICD-10 code and chart review. To this end, most studies attempting to use a specific outcome have focused on the most common, cause-specific diagnoses such as pneumonia10, and to a lesser extent, arterial and venous thrombotic events16. A tradeoff of these more focused analyses has been the loss of sensitivity, which could slow down research on time-sensitive questions.
There are limitations of this study. First, we relied on the inpatient medical team’s assessment of the cause for hospitalization. In some cases, the team’s indicated diagnosis was clearly not related to COVID-19 (e.g., fracture, fall, pain, substance use) but in other cases distinguishing etiology of symptoms was more challenging (e.g., progression of kidney disease). Second, the study population was predominantly White men and included community-dwelling individuals living in the U.S. Third, it is likely that the proportion of all-cause hospitalization (12.3%) was an overestimate given the timeframe under investigation (large Omicron wave). Additional limitations of generalizability extend to Omicron variants beyond BA.1 and BA.2 and after receipt of the bivalent Omicron booster vaccine. Finally, during the observational study period, rapid antigen test became available, so the total number of cases was an underestimate.
Evidence from this study supports use of COVID-19-specific hospitalization in many scenarios instead of all-cause hospitalization in observational surveillance studies. Further, the earlier period (0–15 days) of the outcome was less susceptible to potential measurement bias in contrast to the later period (16–30 days). This report highlights the importance of re-evaluating clinical endpoints in an evolving landscape of viral variants and booster vaccine.
Data availability
The data that support the findings of this study are available from Veterans Health Administration, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Veterans Health Administration. Please contact Dr. Dan Kelly (dan.kelly@ucsf.edu) to request information regarding access for data from this study. More information is available at https://www.virec.research.va.gov. Source data are provided with this paper.
References
Dickerman, B. A. et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. Veterans. N. Engl. J. Med. 386(2), 105–115. https://doi.org/10.1056/NEJMoa2115463 (2022).
Ioannou, G. N., Locke, E. R., Green, P. K. & Berry, K. Comparison of Moderna versus Pfizer-BioNTech COVID-19 vaccine outcomes: A target trial emulation study in the U.S. veterans affairs healthcare system. EClinicalMedicine 45, 101326. https://doi.org/10.1016/j.eclinm.2022.101326 (2022).
Tenforde, M. W. et al. Association between mRNA Vaccination and COVID-19 hospitalization and disease severity. JAMA. 326(20), 2043–2054. https://doi.org/10.1001/jama.2021.19499 (2021).
Dagan, N. et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 384, 1412. https://doi.org/10.1056/NEJMoa2101765 (2021).
Kelly, J. D. et al. Comparative mRNA booster effectiveness against death or hospitalization with COVID-19 pneumonia across at-risk US Veteran populations. Nat. Commun. 14(1), 2976. https://doi.org/10.1038/s41467-023-38503-8 (2023).
Hammond, J. et al. Oral Nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 386(15), 1397–1408. https://doi.org/10.1056/NEJMoa2118542 (2022).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603. https://doi.org/10.1056/NEJMoa2034577 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2035389 (2020).
Bar-On, Y. M. et al. Protection against Covid-19 by BNT162b2 booster across age groups. N. Engl. J. Med. 385(26), 2421–2430. https://doi.org/10.1056/NEJMoa2115926 (2021).
Kelly, J. D. et al. Incidence of severe COVID-19 illness following vaccination and booster with BNT162b2, mRNA-1273, and Ad26.COV2.S vaccines. JAMA 328(14), 1427–1437. https://doi.org/10.1001/jama.2022.17985 (2022).
VA Informatics and Computing Infrastructure. Corporate Data Warehouse (CDW). US Department of Veterans Affairs. Health Services Research & Development. https://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm.
DuVall, S., Scehnet, J. Introduction to the VA COVID-19 shared data resource and its use for research. US department of veterans affairs. Health services research & development. https://www.hsrd.research.va.gov/cyberseminars/catalog-upcoming-session.cfm?UID=3810 (2020).
Centers for Disease Control and Prevention. COVID-19 Information for Specific Groups of People. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html (2022).
McAlister, F. A. et al. The burden of incidental SARS-CoV-2 infections in hospitalized patients across pandemic waves in Canada. Sci. Rep. 13(1), 6635. https://doi.org/10.1038/s41598-023-33569-2 (2023).
Hohl, C. M. et al. Comparing methods to classify admitted patients with SARS-CoV-2 as admitted for COVID-19 versus with incidental SARS-CoV-2: A cohort study. PLoS One 18(9), e0291580. https://doi.org/10.1371/journal.pone.0291580 (2023).
Lo Re, V. et al. Association of COVID-19 vs influenza with risk of arterial and venous thrombotic events among hospitalized patients. JAMA 328(7), 637–651. https://doi.org/10.1001/jama.2022.13072 (2022).
Acknowledgements
This study was supported by Veterans Health Administration Clinical Sciences Research and Development (VA CSR&D) grant I01 CX002417 (Kelly, Keyhani). Dr. Keyhani is also supported by VA Health Sciences Research and Development (VA HSR&D) grants I01 HX002737 and I01 HX003522.
Author information
Authors and Affiliations
Contributions
J. D. K., S. K. conceived and designed the study. J. D. K., S. L., C. C. A., D. M. B. and S. K. contributed to data collection and curation. J. D. K., S. L., C. C. A., D. M. B. and S. K. accessed and verified all data, did data analysis, drafted the first version of the manuscript. All authors assisted in revisions and approval of the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kelly, J.D., Leonard, S., Boscardin, W.J. et al. Re-thinking all-cause COVID-19 hospitalizations as a surrogate measure for severe illness in observational surveillance studies. Sci Rep 14, 14555 (2024). https://doi.org/10.1038/s41598-024-61244-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61244-7
- Springer Nature Limited