Abstract
Glioblastoma (GBM) is a malignant brain glioma characterized by a high number of tumor-associated macrophages (TAMs) within its tissues. These TAMs have a close relationship with tumor grade and prognosis. Targeting TAMs has been identified as a promising therapeutic strategy. However, TAM cells play both tumor-killing and tumor-promoting roles, making them a double-edged sword in the immune environment. The different subtypes of macrophages and their effects on the tumor microenvironment remain poorly understood. This study comprehensively elucidates the immunobiology of glioma-associated macrophages (GAMs), including their origin, classification, molecular mechanisms underlying glioma promotion and inhibition, polarization strategies, targeted therapy for GAMs and the current challenges and perspectives in immune modulation. Further research on macrophage function and mechanism may provide a new immunological basis for treating GBM patients and enhancing the efficacy of glioma immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Macrophages are multifunctional immune cells that exhibit remarkable plasticity and adaptability to diverse tissue environments. The specific niche in which macrophages reside plays a critical role in their homeostatic differentiation. Glioblastoma (GBM) is a highly aggressive brain tumor with a significant population of glioma-associated macrophages (GAMs) within its complex tumor microenvironment (TME). The TME encompasses a diverse array of cellular and molecular constituents that dynamically interact, exerting profound effects on tumor progression and therapeutic response.
GAMs possess a dual function, combating and promoting tumor growth. Usually, they contribute to tumor progression by promoting angiogenesis, facilitating tumor invasion and metastasis, and shaping a microenvironment favorable for tumor growth. Moreover, tumors hijack GAMs through multiple molecular mechanisms, including the regulation of macrophage polarization signals, augmented immunosuppression, epigenetic modifications, and interactions with other components of the immune system.
Considering the essential role of GAMs in tumor development, targeted therapies aimed at GAMs have emerged as promising strategies for glioma treatment. These approaches involve reducing the number of tumor-associated macrophages (TAMs), inducing a phenotypic transformation toward an antitumor M1 polarization state, enhancing their immune regulatory functions, and utilizing various immune modulatory therapies.
This article comprehensively elucidates the intricate immunobiology of GAMs, focusing on their origins, functional classifications and the dynamic interplay between GAMs and the TME. Furthermore, we delve into the potential of targeted therapies of GAMs, discussing strategies to decrease GAMs. By unraveling the complexities of GAMs, this study aims to pave the way for novel immunological approaches in the treatment of GBM, ultimately augmenting the efficacy of glioma immunotherapy.
1.1 Macrophages grow in different tissue environments
1.1.1 Macrophage niche
The macrophage niche refers to the situation in which macrophages regulate their phenotype and function in different tissue environments to maintain the normal structure and function of the tissue. The macrophage niche is mainly determined by the interaction between macrophages and the surrounding cells and molecules (Mass et al. 2023). Guilliams et al. summarized the key features of the macrophage niche, highlighting four crucial aspects. First, the niche provides a physical environment and survival support for macrophages. Second, it provides essential nutritional factors for macrophages, such as CSF-1, IL-32, and CSF-2. Third, it marks the tissue specificity of macrophages by key transcription factors. Finally, macrophages participate in the formation of environmental homeostasis (Guilliams et al. 2020). Macrophages in distinct organs or tissues exhibit unique developmental trajectories, transcriptional programs and life cycles to adapt to their respective niches and fulfill specific functional requirements. These macrophages are called tissue-specific macrophages. They play important roles in various biological processes, such as angiogenesis, adipogenesis, metabolism, and neural function, contributing to organ development, homeostasis, and the immune response (Mass et al. 2023).
1.2 Homeostatic differentiation and steady state
Homeostatic differentiation refers to the process of monocytes adapting to the tissue environment and exerting beneficial functions under normal conditions (Park et al. 2022). During this process, embryonic macrophage precursors or mature monocytes express distinct tissue-specific markers, making them resident tissue macrophages (RTMs) in various tissues, achieving a steady state (Park et al. 2022). In the central nervous system (CNS), RTMs encompass microglia and macrophages located around the dura mater, pia mater, cerebral vessels, and choroid plexus (Kierdorf et al. 2019). Under normal conditions, these RTMs play an essential role in regulating CNS homeostasis (Mundt et al. 2022), including maintaining blood‒brain barrier (BBB) integrity (Ronaldson and Davis 2020), promoting blood flow and cerebrospinal fluid (CSF) drainage and facilitating nutrient transport (Bordon 2023). They also actively engage in waste and impurity clearance, as well as the phagocytosis of senescent apoptotic cells (Chen et al. 2023). Microglia also contribute to the development of the nervous system, such as the establishment of neural circuits (Fujita and Yamashita 2021), the maintenance of synaptic plasticity and the support of oligodendrocyte activity (Zhou et al. 2019). In the face of inflammation or pathological damage, these macrophages not only constitute the first line of defense against pathogens by activating innate immune components but can also regulate adaptive immune responses, presenting necessary antigens to the periphery and suppressing unnecessary immune responses (Muzio et al. 2021). The dysregulation of these responses can precipitate numerous CNS diseases (Kierdorf and Prinz 2017).
Nonhomeostatic differentiation stands in contrast to homeostatic differentiation when disease-related signals are much stronger than steady-state signals, leading to a disruption in monocyte-macrophage differentiation status. This disruption is usually found in the inflammatory state, but in the case of CNS tumors, the opposite effect is observed (Park et al. 2022; Kierdorf and Prinz 2017). Macrophage niches play a regulatory role between these two states by reprogramming transcription in response to different stimulation conditions (Wculek et al. 2022).
When glioma occurs, this homeostasis of macrophages in the CNS is disrupted. Under the action of disease-related signals released by the tumor, the macrophage niche is rewritten as a component of the TME and actively promotes tumor progression (Yin et al. 2017). Under the hijacking of the tumor, RTMs undergo nonsteady-state differentiation toward immune suppression, becoming part of TAMs. Another part of TAMs originates from peripheral bone marrow-derived macrophages (BMDMs) (Hambardzumyan et al. 2016). Consequently, macrophage homeostasis is regulated by the tumor and surrounding environment, creating dynamic heterogeneity.
1.3 GBM
GBM is a malignant brain glioma that is highly invasive, recurrent, and drug-resistant. It is one of the most common and lethal tumors of the central nervous system (Wang et al. 2017). Glioma stem cells (GSCs) are a subgroup of cells with stem cell characteristics that can continuously self-renew and promote GBM differentiation into different subtypes (Xuan et al. 2021). Single-cell RNA sequencing (RNA-seq) and lineage tracing techniques have elucidated the impact of the TME on the transcriptional characteristics of GBM subtypes, consequently influencing the efficacy of tumor treatments (Neftel et al. 2019).
1.4 The TME
The TME is a complex system composed of tumor cells, nontumor cells and molecules, including blood vessels, immune cells, stromal cells, extracellular matrix and other signaling molecules. The components of the TME engage in dynamic interactions with each other, including angiogenesis promotion (Palma et al. 2017; Jiang et al. 2020), the regulation of immune cell polarization, function and migration (Pijuan et al. 2019; Li et al. 2017), modulation of stromal cell phenotype and secretome (Wei et al. 2020; Cai et al. 2021a, b), and important effects on tumor growth, invasion, metastasis and treatment response. It is one of the main factors leading to tumor progression and resistance. The cellular populations of the glioma TME include macrophages/microglia, lymphocytes, astrocytes, oligodendrocytes, and neurons (Nunno et al. 2023). Among these, TAMs emerge as the dominant cell population. TAMs have a symbiotic relationship with tumors and possess strong immunosuppressive properties that promote tumor progression. They enhance the stemness, proliferation, survival, and migration abilities of GSCs while inhibiting adaptive immune responses (Xuan et al. 2021).
Therefore, understanding the changes in macrophage homeostasis is important for studying the glioma-associated TME and identifying potential targets for glioma treatment. This article aims to explore the immunobiological characteristics of GAMs, including their origin, classification, function and regulation mechanisms, while delving into the symbiotic relationship between TAMs and gliomas. Finally, this review will concentrate on several therapeutic approaches that target TAMs, holding substantial promise in the quest for effective glioma treatments.
2 Different origins of GAMs
GAMs in the CNS originate from two primary sources: microglia located in the brain parenchyma and macrophages located around the dura mater, pia mater, brain blood vessels, and choroid plexus (Andersen et al. 2021). In the context of neuroglioma, these two types of cells occupy 30 ~ 50% of the TME (Andersen et al. 2021). However, they differ in their developmental origins, phenotypic markers, gene expression profiles, and functional characteristics.
Microglia are derived from yolk sac progenitors during embryonic development and self-renew throughout life. They express unique phenotypic markers, including TMIGD3, APOC2, SCIN, P2RY12, TREM2, Sall1, Tmem119 and CX3CR1, and exhibit a distinctive gene expression profile that differs from that of other tissue macrophages. In contrast, macrophages in the CNS are derived from bone marrow progenitor cells that migrate across the BBB and differentiate into macrophages in response to local signals. They express different phenotypic markers, such as CD45, CD11b, and F4/80, and their gene expression profile is more closely related to that of other tissue macrophages (Pombo Antunes et al. 2021; Khan et al. 2023).
The traditional method for distinguishing monocyte-derived macrophages and microglia is to use flow cytometry to identify the level of CD45 expression, but microglia in the glioma TME can also exhibit high levels of CD45 (Bian et al. 2020). Currently, TMEM119, Siglec-H, Olfml3 and Sall1 are widely used to distinguish the two (Brandenburg et al. 2020; Andersen et al. 2022). The spatial and temporal distribution of the two in glioma is different. Microglia-derived GAMs are mainly distributed at the tumor margin and infiltration zone, accounting for 82–97% of the cells in the TME of newly diagnosed gliomas, while monocyte-derived GAMs are mainly distributed in the tumor core area and are more prominent in recurrent tumors and necrotic hypoxic areas (Pombo Antunes et al. 2021). Most of the highly invasive macrophages come from the patient’s peripheral blood monocyte-macrophage system (Wang et al. 2022), and the expression of SEPP1 is closely related to tumor recurrence (Pombo Antunes et al. 2021).
3 Functional classification of GAMs
According to the effect of GAMs on tumor growth, they can be roughly divided into two subtypes: antitumor GAMs and protumor GAMs. Antitumor GAMs are similar to M1-type macrophages and express CD68, CD40, CD86 and MHC-II molecules to promote anti-infection and inflammatory immune activation.
They secrete proinflammatory factors such as TNF-α, IL-1β, IL-6, IL-12, IL-23, and NO, which activate the T-cell immune response and induce tumor apoptosis and necrosis (Zhang et al. 2020; Li et al. 2022a, b, c, d, e, f, g). CD169 is considered a marker of proinflammatory macrophages, and M1-like macrophages can produce CXCL9/10 and other T-cell and NK cell chemokines, enhancing the killing effect on tumors (Kim et al. 2022) (Kim et al. 2022). Pro-tumor GAMs are similar to M2-type macrophages, highly expressing CD163, CD204, CD206 and other molecules, secreting anti-inflammatory cytokines such as IL-4, IL-10, IL-13 and TGF-β, and inhibiting the inflammatory response and adaptive immune intensity. M2-like macrophages lack the key expression of T-cell costimulation activation, such as CD86, CD80, and CD40 expression, thus promoting tumor metastasis and immune escape (Zhang et al. 2020; Li et al. 2022a, b, c, d, e, f, g). The number of M2-type macrophages is positively correlated with the pathological grading of WHO tumors (Ding et al. 2014).
For the sake of research convenience, GAMs are generally classified into two categories according to their function, but in fact, the GAM population is a dynamic continuum, with phenotypic and functional heterogeneity and plasticity among its members, and is regulated by various factors in the TME. Several methods have been developed to classify GAMs, and Antunes et al. divided monocyte-derived GAMs (M0) into six types based on single-cell sequencing of human patients and mouse models (Pombo Antunes et al. 2021).
The statement describes the six different types of monocyte-derived GAMs (M0) identified by Antunes et al. based on single-cell sequencing. These include transitional Mo-GAMs, which are in the process of differentiating and express monocyte-related genes but have low expression of mature macrophage markers. GAMs are related to phagocytosis and lipid metabolism and express genes such as GPNMB, LGALS3, and FABP5. GAMs are related to hypoxia and glycolysis and express genes such as BNIP3, ADAM8, MIF, and SLC2A1. GAMs are similar to tissue-resident microglia, expressing genes such as CX3CR1, BIN1, and SCIN, with low expression of SEPP1. GAMs are potentially related to inflammatory activity and highly express genes such as SEPP1, SLC40A1, FOLR2, MRC1, and RNASE1. GAMs with interferon-induced gene expression (Pombo Antunes et al. 2021). Yin et al. also distinguished six different transcriptionally and functionally distinct monocyte/microglia (Mo/Mg) subtypes, as shown in Table 1 (Yin et al. 2022).
Therefore, different GAM subtypes have significant differences, which are reflected in many aspects, such as immune regulation, antigen presentation, and phagocytosis function.
The distribution of GAMs in different molecular subtypes of glioma is different. The proportion of peripheral-derived monocytes in IDH wild-type GBM is significantly higher than that in IDH-mutated grade IV glioma, and their function is mainly inflammation-related (Mo-GAM-inf.), and their tumors show more powerful stemness and richer angiogenesis, while IDH-mutated tumors have a higher proportion of GAMs involved in antigen presentation (Mo/Mg-GAM-APP) (Yin et al. 2022). Different GAM subgroups in the tumor core are related to the overall survival rate of IDH wild-type patients, and studies have found that when the phenotype is CD68−, CD163− and CD206+, it is more conducive to the survival of patients (Zeiner et al. 2019). In IDH-mutated anaplastic astrocytoma, the M2 marker CD168 is negatively correlated with the patient survival rate (Prosniak et al. 2013). In low-grade glioma, GAMs are mainly derived from microglia, and their function is mainly antigen presentation and immune regulation, while high-grade glioma GAMs are mainly derived from peripheral blood monocyte subtypes and are related to immune suppression and inflammation (Andersen et al. 2022). These differences may be related to factors such as chemokines produced by different tumor grades, vascular permeability and necrosis area sizes.
Therefore, further understanding of the TME composition and GAM subtypes of different subtypes of glioma will help to identify appropriate GAM targets and determine accurate immunotherapy.
4 Can GAMs fight against tumors?
M1-like GAMs are a type of immune cell with antitumor activity that are generally considered to have some antitumor ability in the early stages of tumor development. M1 macrophages can directly phagocytose or kill tumor cells through antibody-dependent cytotoxicity. The glycolysis pathway is the basis for M1 macrophages to fight against tumors, and the production of ROS and phagocytosis are highly dependent on glucose supply, while affecting this pathway is the main means for tumors to “hijack” GAMs (Mantovani et al. 2022). M1 macrophages drive adaptive immunity through antigen presentation, promoting T-cell proliferation and IFN-γ secretion (Cendrowicz et al. 2021). Macrophages stimulated by IFN-γ can secrete IL-12, which is a potent antitumor proinflammatory cytokine, and restore the costimulatory activity between GAMs and T cells (Cendrowicz et al. 2021). M1 macrophages can also cause vascular damage and tumor necrosis by producing other proinflammatory factors, such as NO, TNF-α, and IL-12. These factors can activate NK cells, T cells, and B cells, forming effective adaptive immunity (Mantovani et al. 2022; Peng et al. 2020). M1 macrophages can also release various extracellular vesicles (M1EVs), carry antitumor molecules, and regulate the TME (Wang et al. 2022).
As tumor invasion progresses and anti-inflammatory cytokines increase, the latter enhances the suppression of the TME, and GAMs gradually lose all of the above functions, eventually becoming “accomplices” of tumor cells. This process reflects a continuous change in transcription between M1 and M2 macrophages rather than an absolute change (Lanza et al. 2021). Therefore, the general conclusion is that GAMs can coexist with tumors (Cassetta and Pollard 2023). It is currently believed that the immune function of the TME is influenced by PTEN status. The expression level of PTEN is positively correlated with M1 polarization, while reducing the expression of CCL2 and VEGFA inhibits M2 polarization. PTEN cooperates with NHERF-1 to affect macrophage polarization by regulating its expression and membrane localization (Li et al. 2015; Zhou et al. 2022a, b). Sahin et al. reported the role of PTEN in inhibiting macrophage secretion of arginase I (Arg-I), which is considered to be a key enzyme for M2 activation. PTEN regulates Arg-I expression and secretion by inhibiting the Akt signaling pathway, which activates Arg I gene transcription through the transcription factor CCAAT/enhancer binding protein β (C/EBPβ) (Sahin et al. 2014).
5 The promoting effect of GAMs on tumor growth
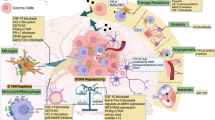
GAMs mainly promote tumor growth in the following aspects, as shown in Fig. 1.
5.1 Promoting angiogenesis
GAMs can secrete various cytokines, including VEGF, FGF1/2, PDGF, and TGF-β, which promote angiogenesis. These cytokines stimulate endothelial cell proliferation, migration, and lumen formation, ultimately providing nutrients and oxygen to tumors. This phenomenon is referred to as “inflammation-driven angiogenesis. “(Cui et al. 2018).
Research by Cui et al. demonstrated that GBM angiogenesis depends on the interaction between TGF-β1 secreted by tumor cells and integrin (αvβ3) on the surface of endothelial cells. Dual blockade of both can effectively inhibit GAM-related angiogenesis and extracellular matrix changes (Cui et al. 2018). Wei et al. found that TNF-α expression leads to vascular endothelial activation in a GBM mouse model, reducing the efficacy of antiangiogenic therapy. Therefore, combining a TNF-α antibody with targeting VEGF can effectively improve treatment efficacy (Wei et al. 2021).
5.2 Promoting tumor invasion and metastasis
GAMs also secrete various proteases, such as MMP2, MMP9, and uPA, that degrade the extracellular matrix and basement membrane. This destroys the normal communication between cells and provides conditions for tumor cell infiltration and migration (Lanza et al. 2021; Quintero-Fabián et al. 2019). In the glioma TME, GM-CSF is upregulated, promoting GAM secretion of large amounts of CCL5, which upregulates MMP2 expression and activity through the calcium-dependent protein kinase CaMKII pathway, achieving directional migration of glioma (Yu-Ju Wu et al. 2020). M2-like GAMs can directly induce tumor cells into the mesenchymal type (CD44+, YKL-40+, MET+, NOTCH+) by mediating the binding of oncostatin M (OSM) to its receptor OSMR or the IF-6 receptor LIFR. This endows tumors with stronger metabolic activity and drug resistance (Hara et al. 2021). After surgical removal of the tumor, GAM-secreted trophic factor (PTN) can even “revive” GSCs (Knudsen et al. 2022). Hypoxia-induced M2 macrophages activate PI3K/Akt/Nrf2 by releasing VEGF, which promotes GBM cell proliferation, epithelial-mesenchymal transition (EMT), GSC characteristics and resistance to TMZ, thereby significantly enhancing tumor invasion ability (Zhang et al. 2022).
5.3 Promoting the formation of a tumor-favorable TME
GAMs secrete various immunosuppressive factors, such as IL-10, TGF-β, PGE2, and IDO, which inhibit the activation and function of T cells, NK cells, and DCs. These factors induce apoptosis of CD4+ and CD8+ T cells and increase the number and activity of Treg cells, enabling tumor cells to escape immune surveillance (Tan et al. 2021a, b). M2-like GAMs can also induce T-cell exhaustion by expressing PD-1 ligands (PD-L1 and PD-L2), CTLA-4 ligands (CD80 and CD86) and VISTA (a potent negative regulator of T-cell function) (Lin et al. 2021). GAMs recruit more immunosuppressive GAMs by releasing IL-11, which activates the STAT3-MYC signaling pathway in GSCs, a process that also depends on the activation of PI3K-γ (Li et al. 2021).
5.4 Promoting tumor cell growth and metabolism
GAMs can interact with tumor cells through direct contact or indirect secretion of factors, affecting tumor cell proliferation, differentiation, metabolism, apoptosis and stem cell characteristics. TGFβ-1 is a protein secreted by GAMs that promotes the growth and stemness of GSCs via the integrin avβ5-Src-Stat3 signaling pathway (Xiao et al. 2022). Macrophage-released osteopontin (OPN)/SPP1 activation of CD44 is critical for tumor stemness, and SPP1 interaction with CD44 can activate downstream pathways of MES-like transformation in glioma cells, increasing their invasiveness and drug resistance (He et al. 2021). This process can also regulate the TME to increase the number of M2-like GAMs while inhibiting CD4+ and CD8+ T-cell activity (Pietras et al. 2014) (Zhao et al. 2020). M2-like macrophages promote glycolysis in tumor cells by releasing IL-6 and IL-1β, phosphorylating PGK1 and GPD2, etc., increasing tumor cell lactate production and ATP levels and enhancing tumor energy metabolism (Zhang et al. 2018; Lu et al. 2020). Overexpression of Chitinase-3 like-protein-1 (CHI3L1) in TME often indicates poor prognosis, which is widely expressed by macrophages and others, binds to its receptors IL-13Ra2, TMEM219, Gal-3, CRTH2 and CD44, activates NF-κB and other pathways, and promotes glioma proliferation (Zhao et al. 2020).
6 Tumor “hijacking” of GAMs
Tumor “hijacking” of GAMs refers to the tumor altering the phenotype and function of GAMs through various mechanisms, making them a phenotype that is conducive to tumor cell survival. These mechanisms are shown in Fig. 2.
Glioma GAM imbalance mechanism: (1) Immunosuppressive molecules, such as TGF-β and IL-10, promote GAM transformation to the M2 type, reduce the immune activity of macrophages, and inhibit the killing effect of immune cells on tumor cells (2) Cytokines, such as CSF-1 and VEGF, recruit and activate GAMs to tumor tissues and promote the transformation of GAMs to the M2 type, promoting the growth of blood vessels. (3) miRNA: In glioma, the expression of certain miRNAs is water flat and related to the polarity of GAMs. For example, miR-142-3p can promote the conversion of GAMs to the M1 type, while miR-511 can promote the conversion of GAMs to the M2 type
6.1 Regulating macrophage polarization signals
Tumor cells can secrete various polarization factors, such as CSF-1, IL-4, IL-10, IL-13, and IL-34, which can induce macrophage polarization to M2 (Tong et al. 2021). Different polarization mechanisms are shown in Fig. 3. Glioma cells can release various miRNAs through exosomes, including miR-1246, miR-155-3p, miR-10b-5p, miR-32 and miR-3591-3p. These miRNAs can be taken up by GAMs and affect the expression of genes such as IRF1, PTEN, CBLB, and MAPK1, inhibiting the corresponding signaling pathways IRF1/IFN-β, PTEN/PI3K/AKT, JAK2/STAT3, and MAPK, thereby promoting GAMs to polarize to the M2 type (Qian et al. 2020; Xu et al. 2021; Li et al. 2022a, b, c, d, e, f, g; Bao and Li 2019; Shi et al. 2020; Pan et al. 2022).
The polarization of GAMs: As a duplicitous in the immune environment, GAM cells play a two-way role: tumor killing effect vs. pro-tumor effect (M1: inflammation, phagocytosis, attack; M2: immunosuppression, fibrosis formation, tissue repair, angiogenesis). GAMs are continuous: the state of continuous transition between M1 and M2 morphologies, and the proportion of each morphology depends on the type and concentration of different secretions in the receiving tumor environment
High-grade gliomas often overexpress EWSR1, mediating the production of circular RNA circNEIL3 and affecting IGF2BP3 stability, thereby affecting the polarization of M2-like GAMs and releasing IL-10, GF-β, and other immunosuppressive factors (Li et al. 2022a, b, c, d, e, f, g). In GBM, the β2-microglobulin (B2M) subunit of MHC-II is activated by TGFβ-1 and promotes M2 transformation through SMAD and PI3K/AKT (Li et al. 2022a, b, c, d, e, f, g; Ma et al. 2021). GSCs have the ability to regulate CD90 expression and increase macrophage immune suppression through the USF1/CD90 axis while enhancing their own stemness (Zhou et al. 2022a, b). ARS2/MGAL signaling in GSCs can also promote GAM self-renewal and M2 polarization (Yin et al. 2020). TERM2 expression is increased in glioma and is closely related to the enrichment of GAMs, especially M2-like macrophages. It can also inhibit the macrophage response to TLR ligands (Yu et al. 2022).
6.2 Enhanced immunosuppression of the TME
Glioma cells release the secretory peptides SLIT2 and OPN, which bind to ROBO receptors and integrin ITGαvβ5 on the surface of macrophages, respectively, activating the PI3K-γ signaling pathway, mediating macrophage chemotaxis to the TME, and expressing cytokines to promote tumor growth and angiogenesis (Geraldo et al. 2021; Wei et al. 2019). ATRX-deficient IDH-mutant gliomas exhibit more pronounced astrocytic characteristics while reducing the binding of CCCTC-binding factor (CTCF) to DNA. This disrupts the expression of chemokines such as GFAP, CXCL12, and CXCL14, mediating more GAM infiltration and remodeling the immune microenvironment (Babikir et al. 2021). MET-overexpressing tumors recruit more immunosuppressive GAMs by activating MET-STAT-PD-L1 (Wang et al. 2021). The nuclear function of tumor-secreted IL-33 can induce macrophages to produce a series of chemokines that recruit and activate circulating monocytes, such as CCL-2, CCL-5 and CXCL10, creating a glioma ‘habitable environment’ (Boeck et al. 2020). Tumor cells can consume large amounts of oxygen and glucose, resulting in a state of hypoxia and hypoglycemia in the tumor microenvironment. This state can activate HIF-1α and AMPK signaling pathways in macrophages, promoting M2 polarization (Xiao et al. 2022). RNA regulatory factor (HuR) is a key factor in regulating tumor immune suppression. Knockout of HuR animal models showed tumor shrinkage, relatively increased M1-like GAMs, reduced PD-L1 expression, increased infiltration of CD4+ T cells (including Th1 and CTL), and reduced tumor-associated polymorphonuclear myeloid-derived suppressor cells (Wang et al. 2019). Matrix remodeling-associated protein 8 (MXRA8) is a novel prognostic indicator that may be involved in ferroptosis. MXRA8 expression in gliomas is significantly higher than that in normal brain tissue, and high expression of MXRA8 is associated with poor prognosis (Xu et al. 2022a, b). MXRA8 is related to various immune suppressive factors, such as TGF-β1, IL-10, PD-L1 and CTLA4. Studies have found that knocking down this gene can reduce M2 infiltration and rescue glioma progression (Xu et al. 2022a, b).
6.3 Regulating the immune microenvironment by epigenetic modification
Epigenetic modification is the process of regulating gene expression without changing the DNA sequence. Common epigenetic dysregulation in tumors includes mutations and abnormal expression of epigenetic modification enzymes and changes in the levels of related cofactors, which alter the structure and dynamics of chromatin, leading to changes in gene expression and ultimately promoting tumor initiation and progression (Gangoso et al. 2021). Epigenetic modification of gliomas greatly increases the opportunity to regulate the immune microenvironment. Mesenchymal-like GBM cells promote immune escape under host defense by epigenetic modifications, including loss of CpG island methylation associated with IRF1, CCL2, and IRF8 (Gangoso et al. 2021). This immune escape mainly involves increased bone marrow-derived GAMs and T-cell exhaustion (Goswami et al. 2020; Mohme and Neidert 2020). Under hypoxic conditions, glioma cells express ALKBH5. This enzyme can remove N6-methyladenosine (m6A) modification on the long noncoding RNA (lnc-RNA) NEAT1, which can increase CXCL8/IL8 expression by inhibiting SFPQ and increase chemotaxis to GAMs (Dong et al. 2021; Wei et al. 2022).
6.4 Regulating the interaction of macrophages with other immune components
There are various immunosuppressive factors in the tumor microenvironment. M2 macrophages inhibit the activation of CD8 + T cells and cDC1s by expressing molecules such as PD-L1, CD47 and ARG1 and secreting factors such as IL-10 and TGF-β while reducing the efficacy of targeting PD-1 (Zhu et al. 2020; Lee et al. 2021). Hu et al. found that gliomas escape the phagocytosis of GAMs by expressing high levels of LRIG2, which increases the expression of CD47 on M2 macrophages (Hu et al. 2022).
Canine uric acid produced by GBM cells activates AHR in GAMs, promoting CCR2 expression. At the same time, AHR promotes the expression of ectonucleotidase CD39 in GAMs, which cooperates with CD73 to produce adenosine, leading to CD8+ T-cell dysfunction (Takenaka et al. 2019). At the same time, AHR promotes the expression of ectonucleotidase CD39 in GAMs, which cooperates with CD73 to produce adenosine, leading to CD8+ T-cell dysfunction (Takenaka et al. 2019). Recurrent gliomas encode temozolomide-associated lnc-RNA (lnc-TALC), which activates the p38-MAPK signaling pathway to promote GAM release of complement C5/C5a after binding to ENO1, thereby promoting tumor cell DNA repair. Therefore, combined immunotherapy targeting C5a can overcome lnc-TALC-mediated TMZ resistance (Boeck et al. 2020).
7 Targeted GAM therapy
Targeted GAM therapy refers to the use of drugs or biological agents to intervene in the number, phenotype and function of GAMs, thereby inhibiting tumor growth and metastasis. The mechanism of targeted GAM therapy is visualized in Fig. 4.
Several targeted GAMs therapies. A: The CSF1/CSF1R signaling axis is overexpressed in high-grade gliomas and is associated with poor prognosis. Monoclonal antibodies or small molecule inhibitors targeting CSF-1/CSF1R can block CSF1R signaling. B: Immunovirotherapy: Oncolytic viruses may dissolve GBM, releasing tumor antigens and danger signals, or induce polarization of GAMs into M1 by upregulating PD-L1, TLR, or NF-kB, either due to therapeutic factors carried by OV such as GM-CSF, IFN-γ, or IL-12. C, D: Two classic immune checkpoint therapies: PD-1 antibodies, on one hand, restore the function of T cells, inhibit tumor growth and metastasis. On the other hand, even in the case of CD8+ T cell exhaustion, PD-1 antibodies can still induce GAMs to polarize towards the M1 phenotype, producing anti-tumor effects. CD47 sends a ‘do not eat me’ signal to macrophages, preventing them from engulfing tumors. The combination of CD47 and CD24 antibodies effectively activates the bone marrow immune response, kills cancer cells, and simultaneously interrupts the malignant cycle caused by GSCs revival
7.1 Reduce the number of GAMs
By inhibiting the survival or recruitment of macrophages, the number of GAMs in the TME can be reduced, thereby alleviating the protumor effect of the TME.
7.1.1 Targeting CSF-1 therapy
Macrophages depend on CSF-1 for differentiation and survival, and its paracrine signaling pathway plays a key role in the symbiotic relationship between glioma cells and GAMs (Pyonteck et al. 2013). Therefore, inhibiting the CSF-1/CSF-1R signaling pathway is a representative strategy for targeting GAM depletion, which blocks the migration and differentiation of peripheral blood monocytes into tumor tissues and significantly enhances the efficacy of other therapeutic measures (Pombo Antunes et al. 2021; Akkari et al. 2020). This therapy has also achieved some results in medulloblastoma models (Tan et al. 2021a, b). The efficacy of CSF-1 inhibitors depends on whether glioma cells are driven by PDGFR or RAS genes (Rao et al. 2022). The efficacy of CSF-1 inhibitors depends on whether glioma cells are driven by PDGFR or RAS genes. PDGFR-driven gliomas induce the proliferation and activation of microglia, which depend on the CSF-1 signaling pathway for differentiation and survival. RAS-driven gliomas have GAMs rich in proinflammatory and proangiogenic signals, which can maintain survival through other pathways (such as GM-CSF and IFN-γ), even when CSF-1R is inhibited (Rao et al. 2022). Therefore, combined antiangiogenic therapy can significantly improve the efficacy of CSF-1 inhibitors against RAS tumors (Rao et al. 2022). However, Antunes et al. used a mouse model of glioma and showed that oral administration of the CSF1R inhibitor PLX3397 did not attenuate monocyte infiltration into GBM but rather increased the percentage of monocytes in tumors. They believe that the mechanism of targeting CSFR is to block monocyte differentiation into GAMs (Pombo Antunes et al. 2021).
Several studies have reported methods to observe the efficacy of targeting CSF-1 by imaging methods. Foray et al. developed a method to noninvasively observe the efficacy of a CSF-1R inhibitor using 18 F-DPA-714 by PET/MRI, which showed that macrophage infiltration was significantly reduced and tumor volume was significantly reduced after treatment (Foray et al. 2022). Li et al.‘s T2-weighted MRI imaging methods can also detect this process (Li et al. 2022a, b, c, d, e, f, g).
7.1.2 Other therapies to reduce GAMs
CCR2 is a key molecule for the chemotaxis and activation of macrophages (Boissonnas and Combadière 2022). Silva et al. reported an inhibitor that targets and inhibits glutamyl cyclotransferase-like protein (QPCTL), which can inhibit the N-terminal degradation of CCR2 and CCL7, preventing macrophages from being released from the bone marrow and migrating to tumor tissues and limiting the recruitment of GAMs (Barreira da Silva et al. 2022). The targeting CCR2 inhibitor CCX872 also showed similar effects in glioma and other tumor models (Tu et al. 2020; Flores-Toro et al. 2020). PI3K is a key enzyme in tumor signal transduction, and its subtype PI3Kγ plays an important role in GAM polarization and migration. Inhibition or knockout of PI3Kγ can reduce the number of GAMs in the TME and enhance the chemotherapeutic effect of TMZ (Li et al. 2021).
7.2 Phenotype of transformed GAMs
In theory, activating M1 polarization signals or inhibiting M2 polarization signals can achieve GAM repolarization, and there are currently multiple ways to deliver regulatory factors to the TME to exert effects.
7.2.1 Promotion of M1 polarization
On the one hand, activating TLR, IFN-γ, IL-12, STAT1 and other signaling pathways can promote GAM polarization to the M1 type (Birocchi et al. 2022). Xue et al. found that chlorogenic acid (CHA) affects the STAT1 and STAT6 signaling pathways, promoting macrophages to transform into the M1 phenotype and inhibiting tumor growth in animal models (Xue et al. 2017). Ginsenoside Rg3 is a plant sterol with antitumor activity. Zhu et al. developed liposomes coloaded with Rg3 and paclitaxel (Rg3-PTX-LPs), which can increase the ratio of M1/M2 macrophages, reduce the expression of the M2 markers CD206 and Arg-1, and increase the expression of the M1 markers iNOS and TNF-α, possibly through the activation of STAT1 (Zhu et al. 2021). Li et al. found that high mobility group protein B1 (HMGB1) is a key regulator between glioma cells and M1 polarization. HMGB1 induces macrophages to polarize into M1 through the RAGE-NFκB-NLRP3 inflammasome pathway and enhances tumor cell sensitivity to TMZ (Li et al. 2022a, b, c, d, e, f, g). Magnolol and disulfiram/copper (DSF/Cu) coloaded peptide-modified liposomes can target the TME, induce apoptosis through ROS and autophagy inhibition, effectively inhibit angiogenesis, repolarize GAMs into the M1 phenotype, and enhance T-cell and NK cell antitumor immune responses (Zheng et al. 2020; Li et al. 2022a, b, c, d, e, f, g). Birocchi made engineered hematopoietic stem cells (HSCs), which can improve GAM immune suppression by releasing IFN-α and IL-12 in the TME, improving the survival rate of glioma model mice, and this study is currently undergoing a phase I clinical trial in Italy (Birocchi et al. 2022). In vitro transcription mRNA (IVT mRNA) is a novel potential targeted drug. Zhang et al. developed an IVT mRNA nanocarrier for GAM repolarization. After entering the TME, the carrier polymer is hydrolyzed, releasing mRNA that is transcribed by ribosomes into IRF5 and IKKβ proteins, which then activate M1 polarization-related signaling pathways, expressing IL-12, IFN-γ and TNF-α and other inflammatory cytokines, while inhibiting M2 polarization-related immune suppression and protumor factors such as IL-6, Serpinb2 and CCL11 (Zhang et al. 2019). These approaches show promise in achieving GAM repolarization and improving the efficacy of immunotherapy in gliomas and other cancers. However, further research is needed to optimize delivery methods and dosing strategies to maximize therapeutic benefits while minimizing potential side effects.
7.2.2 Inhibition of M2 polarization
On the other hand, inhibiting signaling pathways such as IL-4, IL-10, IL-13, STAT3 and STAT6 can suppress GAM polarization to M2 (Yang et al. 2022a, b). Carnosic acid (CA) is a natural compound derived from rosemary that can inhibit the Wnt/β-catenin-WISP1 signaling pathway. WISP1 is a protein secreted by GSCs that can promote GSC survival and proliferation and affect immune cells in the tumor microenvironment (Tao et al. 2020). CA reduces WISP1 expression, decreases the M2 phenotype, and prolongs survival time in mouse models (Tao et al. 2020). Yang et al. recently developed the nanodrug SPP-ARV-825, which works by inhibiting IFR4 promoter transcription, affecting STAT3/6 and AKT phosphorylation, and thereby suppressing M2 polarization (Yang et al. 2022a, b). MK-8931 was originally a BACE1 inhibitor for treating Alzheimer’s disease and has entered phase III clinical trials. It was recently shown to alter the GAM phenotype by inhibiting the IL6/STAT3 pathway and synergistically enhance radiotherapy efficacy in animal models (Zhai et al. 2021). Palbociclib is a CDK6 inhibitor that can reduce M2 polarization markers. This substance can also inhibit glioma growth by various methods, such as reducing lncRNA SNHG15 expression and increasing miR-627-5P (Li et al. 2019).
7.2.3 miRNA therapy
miRNA is a small noncoding RNA that regulates mRNA degradation or translation by binding to mRNA with incomplete base pairing (Guo et al. 2019). miRNA therapy provides a highly selective method to achieve precise modulation of the immune microenvironment. miR-124 is the most abundant miRNA in the CNS and is involved in neural development and functional regulation (Xu et al. 2022a, b). Several studies have found that miR-124 vectors have therapeutic potential by inhibiting M2 polarization through targeting the STAT3 and C/EBPα pathways and alleviating radiation-induced cognitive dysfunction (RICD) to some extent after radiotherapy (Yin et al. 2020; Leavitt et al. 2020; Romano et al. 2020). miR-22 is a significantly downregulated miRNA in glioma cells and GAMs that can inhibit the NF-κB signaling pathway by targeting HDAC6, thereby enhancing macrophage phagocytosis, antigen presentation and T-cell activation abilities and inhibiting glioma growth and progression (Tu et al. 2022). Currently, some studies have explored the possibility of miR-22 involvement in therapy, including using nanoparticles or macrophages as carriers to deliver miR-22 to tumor sites or combining with chemotherapy or radiotherapy to enhance therapeutic effects (Müller Bark et al. 2020; Kirstein et al. 2020).
7.3 Improving the immune regulatory function of GAMs
The function of GAMs depends on various expressed receptors and secretions, and regulating them can regulate their immune function.
7.3.1 Antiangiogenic therapy
Inhibiting angiogenic factors secreted by GAMs, such as VEGF, bFGF, and PDGF, or blocking their binding to corresponding receptors can cut off the nutrient supply of tumors (Gilbert et al. 2017). Bevacizumab is a monoclonal antibody against VEGF-A that can inhibit angiogenesis and tumor growth (Ahir et al. 2020) and significantly improved PFS-6 in phase III clinical trials (Gilbert et al. 2014). The VEGF receptor tyrosine kinase inhibitor cediranib also showed significant efficacy in a GBM phase II clinical trial (Batchelor et al. 2010). Inhibiting proteases such as MMPs can reduce tumor invasion and metastasis (Peng et al. 2022), and potential MMP inhibitors with therapeutic value include DX-2400, AB0041, AB0046 and GS-5745, which are currently in clinical trials (Fields 2019; Levin et al. 2017). Glioma cells can stimulate GAMs to produce TNF-α by secreting IL8 and CCL2, which then mediates endothelial activation (Wei et al. 2021). This is the main cause of antiangiogenic therapy failure, and inhibiting TNF-α effectively blocks endothelial activation and increases the survival rate in model mice (Wei et al. 2021).
7.3.2 Immune checkpoint regulation
Immune checkpoints are a class of molecules that can regulate the intensity and duration of immune responses, and tumor cells often use them to evade immune surveillance and clearance. CD47 and CD24 are two immune checkpoint molecules that send “don’t eat me” signals to macrophages, preventing macrophage phagocytosis of tumors (Barkal et al. 2019). The combined use of CD47 and CD24 dual antibodies effectively activates bone marrow immunity, significantly kills cancer cells, and interrupts the vicious cycle caused by GSC revival (Wu et al. 2021). Etomoxir combined with a CD47 monoclonal antibody inhibits CD47 expression and function and restores the macrophage phagocytosis ability of tumors (Jiang et al. 2022).
B7-H3 is also an immune checkpoint molecule that can inhibit macrophage phagocytosis of tumor cells by interacting with Siglec-10 on the surface of macrophages and is associated with poor prognosis of tumors (Huang et al. 2020). B7-H3 promotes glioma progression through the JAK2/STAT3/Slug signaling pathway, and the STAT3 inhibitor napabucasin can significantly inhibit B7-H3-mediated in vivo and in vitro tumor growth (Tang et al. 2019). CXCL14 is a potential immunotherapy adjuvant factor that can increase MHCI expression on the surface of gliomas, chemoattract CTL cells in vivo and in vitro, and inhibit glioma growth in model mice but is downregulated in IDH-mutated gliomas (Kumar et al. 2022). High B7-H3 expression also inhibits CXCL14 signaling; therefore, targeting both B7-H3 and CXCL14 can achieve optimal therapeutic effects (Kumar et al. 2022).
PD-1 antibody is the most representative immune checkpoint inhibitor, and blocking the PD-1 and PD-L1 interaction can restore T-cell function and inhibit tumor growth and metastasis (Cai et al. 2019). However, the efficacy of PD-1 antibodies does not depend solely on T cells, and recent studies have found that innate immunity also plays an important role in this process (Cai et al. 2019; Fierro et al. 2022). In the case of CD8+ T-cell depletion, an anti-PD-1 antibody can still induce GAM polarization to the M1 phenotype by producing antitumor effects (Liu et al. 2023). M1-like GAMs express high levels of PD-L1, providing more targets for PD-1 antibodies (Ruan et al. 2021). VEGF not only promotes angiogenesis but also induces tumor cells and GAMs to overexpress PD-L1 by activating the VEGFR1 signaling pathway (Liu et al. 2023). However, this process can be inhibited by soluble VEGFR1 (sVEGFR1). When the PD-L1 antibody blocks PD-L1, it can promote GAMs to secrete more sVEGFR1, suggesting the potential of immune checkpoint inhibitors combined with antiangiogenic therapy (Liu et al. 2023; Ruan et al. 2021). Dendritic cell vaccines can induce tumor-specific T-cell immunity but lead to upregulation of PD-L1 on macrophages (Antonios et al. 2017). When anti-PD-1 monoclonal antibodies and CSF-1R inhibitors are used in combination with dendritic cell vaccines, they can significantly prolong mouse survival, increase T-cell number and activity, and produce long-term memory immunity (Antonios et al. 2017). NAMPT inhibitors can reduce NAD levels in GBM cells and change the immune status in the tumor microenvironment, leading to upregulation of PD-L1, reduction in M2-type macrophages, and inhibition of tumor cell growth (Li et al. 2020). IL-6 is an immunosuppressive cytokine that induces PD-L1 expression through the STAT3 pathway (Lamano et al. 2019). In a mouse GBM model, IL-6 transduction inhibition reduced PD-L1 expression on bone marrow-derived macrophages, reduced tumor growth, and prolonged mouse survival. This effect is CD8+ T-cell-dependent and has a synergistic effect with anti-PD-1 monoclonal antibody treatment (Lamano et al. 2019).
7.3.3 Oncolytic viruses
Oncolytic viruses (OVs) can directly lyse tumor cells or induce antitumor immune responses (immunotherapy). OV can affect the phenotype and function of GAMs in various ways. On the one hand, OV can induce GAM polarization to the M1 type, thereby producing antitumor immune responses (Sanchez Gil and Rabkin 2022). This effect may be due to GAM death caused by OV infection, releasing tumor antigens and danger signals, or due to OVs carrying ‘weapons’, such as GM-CSF, IFN-γ or IL-12 (Sanchez Gil and Rabkin 2022). On the other hand, OV can also produce antiviral responses by upregulating PD-L1, TLR or NF-kB, limiting virus replication and spread (Blitz et al. 2022; Christie and Chiocca 2022). Kiyokawa et al. developed an oncolytic adenovirus (ICOVIR17) expressing hyaluronidase (PH20), which promotes M1 polarization through NF-kB while degrading hyaluronic acid (HA) in the TME, showing broader efficacy (Kiyokawa et al. 2021). Researchers combined the use of oncolytic virus expressing IL-12 (G47Δ-mIL12), PD-1 antibody and CTLA-4 antibody. On the one hand, oncolytic viruses disrupt tumor cells to release antigens and promote antigen presentation. On the other hand, M1 cells activated by IL-12 further enhance CD8+ T-cell killing activity while reducing Treg number and function. The treated animal models showed signs of tumor eradication (Saha et al. 2017).
7.3.4 Targeting CD40 therapy
CD40 is a costimulatory molecule expressed on GAMs that can bind to the ligand CD40L, activate GAM immune function, and induce tumor cell apoptosis and the immune response. Currently, two clinical trials are evaluating the efficacy of humanized IgG1CD40 monoclonal antibody treatment for GBMs (NCT02304393, NCT04164901). Studies have found that IL-6 can induce GAMs to express CD40 through two signaling pathways, STAT3 and HIF-1α, thereby counteracting the immunosuppressive effect of IL-6 on GAMs. Therefore, targeting both IL-6 and CD40 is a potential therapeutic strategy (Yang et al. 2021).
7.3.5 Other GAM-related immune regulatory therapies
Ferroptosis is the main mode of cell death for glioma cells, but it also enhances M2 activity, leading to immune suppression and tumor growth (Cai et al. 2021a, b). Therefore, Liu et al. demonstrated that patients with weak ferroptosis activity had better efficacy with PD-L1 antibody in animal models and the IMvigor210 clinical immunotherapy cohort (Liu et al. 2022). Recently, it was found that NH1 inhibitors restored GAM mitochondrial oxidative phosphorylation (OXPHOS) function, increased GAM glucose uptake and mitochondrial activity, and reduced aerobic glycolysis, thereby promoting cellular immunity and enhancing TMZ and PD-1 antibody efficacy (Hasan et al. 2021). Yang et al. prepared zinc cyclic di-AMP nanoparticles (ZnCDA), which targeted STING binding of macrophages and activated the TBK1/IRF3 and IKK/NF-κB signaling pathways, thereby inducing the production of IFN-I and other inflammatory factors, stimulating macrophages to express MHC-II molecules and costimulatory molecules, enhancing antigen presentation ability, and promoting T-cell activation and proliferation (Yang et al. 2022a, b).
8 Discussion and conclusions
This review discusses the immunobiological characteristics of GAMs, including their origin, classification, function and regulation mechanisms, and discusses potential therapeutic strategies. GBM is a malignant brain glioma with high invasiveness, recurrence and drug resistance and is one of the most common and fatal tumors in the CNS (Wang et al. 2017). The impact of GBM on TME homeostasis is multifaceted and intricate. By promoting angiogenesis, immune evasion, invasion, metastasis, and drug resistance, GBM exerts an influence on various components of the TME, thus creating a conducive environment for tumor growth, progression, and responses to treatment. GAMs are the major cell population in the TME and have an extensive symbiotic relationship with the tumor. They can promote the stemness of GSCs, enhance cell proliferation, survival, and migration, and suppress T-cell-mediated immune responses, thereby promoting tumor progression (Xuan et al. 2021).
The classification of GAMs remains a subject of intense debate within the academic community. Currently, there is widespread recognition that GAMs exhibit a heterogeneous and plastic range of phenotypes (Pombo Antunes et al. 2021; Yin et al. 2022). However, consensus on their identification and classification has not yet been achieved. Although researchers have discovered various biomarkers to delineate subsets and functions of GAMs, these classification methods are often overly complex and have not provided effective guidance for foundational experiments. Consequently, current experimental approaches often rely on a binary categorization of GAMs into two subtypes: tumor-suppressive GAMs and tumor-promoting GAMs. In the context of GBM, GAMs primarily exhibit immunosuppressive characteristics. The tumor-promoting effects of GAMs in GBM are evident in several crucial aspects. First, they actively promote angiogenesis, thereby facilitating the formation of new blood vessels to sustain tumor growth (Cui et al. 2018; Wei et al. 2021). Second, GAMs play a role in facilitating tumor invasion and metastasis, enabling the spread of cancer cells to distant sites (Xiao et al. 2022; He et al. 2021; Pietras et al. 2014; Zhang et al. 2018; Lu et al. 2020; Zhao et al. 2020). Additionally, GAMs create a TME that is conducive to tumor growth and progression (Tan et al. 2021a, b; Lin et al. 2021; Li et al. 2021). Finally, GAMs enhance tumor cell proliferation and metabolism, further contributing to the aggressive nature of GBM (Xiao et al. 2022; He et al. 2021; Pietras et al. 2014; Zhang et al. 2018; Lu et al. 2020; Zhao et al. 2020). To ensure their survival, tumors employ various mechanisms to modify the phenotype and function of GAMs, effectively transforming them into phenotypes that support tumor cell survival. Therapeutic strategies targeting GAMs encompass a range of approaches, such as inhibiting their infiltration or polarization and reshaping their phenotype or function (Birocchi et al. 2022; Xue et al. 2017; Zhu et al. 2021; Li et al. 2022a, b, c, d, e, f, g; Zheng et al. 2020; Zhang et al. 2019). These strategies can be employed individually or in combination with other treatment modalities to improve the prognosis of GBM patients. By understanding the complex role of GAMs and their contribution to tumor progression, researchers can develop more effective therapeutic interventions that specifically target these cells, ultimately leading to improved outcomes for GBM patients.
Currently, there are several limitations to the targeted treatment strategies for glioma by focusing on GAMs. First, GAMs exhibit extensive heterogeneity and plasticity, necessitating precise timing and a clear understanding of the individual TME status for effective targeted therapy. Second, gliomas themselves demonstrate significant heterogeneity, requiring further investigation into the interactions between different types of GAMs and each subtype of glioma, as well as exploring their dynamic changes over time. GAMs likely play distinct roles in glioblastoma subtypes, grades, and mutational states, necessitating personalized approaches to optimize targeted GAM therapy. Third, although mice and human gliomas have some conservation of immune components, it is still difficult to use animal models to study the relationship between the glioma microenvironment and macrophages. For example, there is an isolation barrier between transplanted glioma tissue and normal brain tissue in animal models, but human primary brain tumors exhibit infiltrative growth. Therefore, using primary mouse glioma models can more accurately simulate the immune microenvironment of human gliomas. Fourth, most of the therapeutic strategies targeting GAMs are still in preclinical or early clinical stages, and more clinical trials are needed to evaluate their safety, efficacy and tolerability. We hope that more effective methods can be found in the future to regulate the immune microenvironment of gliomas to enhance the efficacy of immunotherapy.
Availability of data and materials
This article does not contain any data materials; therefore, this section is not applicable.
References
Ahir BK, Engelhard HH, Lakka SS. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol Neurobiol. 2020;57(5):2461–78.
Akkari L, Bowman RL, Tessier J, Klemm F, Handgraaf SM, de Groot M, et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med. 2020;12(552):eaaw7843.
Andersen BM, Faust Akl C, Wheeler MA, Chiocca EA, Reardon DA, Quintana FJ. Glial and myeloid heterogeneity in the brain tumour microenvironment. Nat Rev Cancer. 2021;21(12):786–802.
Andersen JK, Miletic H, Hossain JA. Tumor-associated macrophages in Gliomas-basic insights and treatment opportunities. Cancers (Basel). 2022;14(5):1319.
Antonios JP, Soto H, Everson RG, Moughon D, Orpilla JR, Shin NP, et al. Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro Oncol. 2017;19(6):796–807.
Babikir H, Wang L, Shamardani K, Catalan F, Sudhir S, Aghi MK, et al. ATRX regulates glial identity and the tumor microenvironment in IDH-mutant glioma. Genome Biol. 2021;22(1):311.
Bao L, Li X. MicroRNA-32 targeting PTEN enhances M2 macrophage polarization in the glioma microenvironment and further promotes the progression of glioma. Mol Cell Biochem. 2019;460(1–2):67–79.
Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–6.
Barreira da Silva R, Leitao RM, Pechuan-Jorge X, Werneke S, Oeh J, Javinal V, et al. Loss of the intracellular enzyme QPCTL limits chemokine function and reshapes myeloid infiltration to augment tumor immunity. Nat Immunol. 2022;23(4):568–80.
Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–23.
Bian Z, Gong Y, Huang T, Lee CZW, Bian L, Bai Z, et al. Deciphering human macrophage development at single-cell resolution. Nature. 2020;582(7813):571–6.
Birocchi F, Cusimano M, Rossari F, Beretta S, Rancoita PMV, Ranghetti A, et al. Targeted inducible delivery of immunoactivating cytokines reprograms glioblastoma microenvironment and inhibits growth in mouse models. Sci Transl Med. 2022;14(653):eabl4106.
Blitz SE, Kappel AD, Gessler FA, Klinger NV, Arnaout O, Lu Y, et al. Tumor-associated Macrophages/Microglia in Glioblastoma Oncolytic virotherapy: a double-edged sword. Int J Mol Sci. 2022;23(3):1808.
Boissonnas A, Combadière C. Modulating the tumor-associated macrophage landscape. Nat Immunol. 2022;23(4):481–2.
Bordon Y. Macrophages bordering the brain parenchyma regulate the flow of cerebrospinal fluid. Nat Rev Immunol. 2023;23(1):3–3.
Brandenburg S, Blank A, Bungert AD, Vajkoczy P. Distinction of Microglia and macrophages in Glioblastoma: close relatives, different tasks? Int J Mol Sci. 2020;22(1):194.
Cai J, Qi Q, Qian X, Han J, Zhu X, Zhang Q, et al. The role of PD-1/PD-L1 axis and macrophage in the progression and treatment of cancer. J Cancer Res Clin Oncol. 2019;145(6):1377–85.
Cai X, Yuan F, Zhu J, Yang J, Tang C, Cong Z, et al. Glioma-associated stromal cells stimulate glioma malignancy by regulating the tumor immune microenvironment. Front Oncol. 2021;11:672928.
Cai Y, Liang X, Zhan Z, Zeng Y, Lin J, Xu A, et al. A ferroptosis-related gene prognostic index to predict Temozolomide sensitivity and immune checkpoint inhibitor response for Glioma. Front Cell Dev Biol. 2021;9:812422.
Cassetta L, Pollard JW. A timeline of tumour-associated macrophage biology. Nat Rev Cancer. 2023;23(4):238–57.
Cendrowicz E, Sas Z, Bremer E, Rygiel TP. The role of macrophages in cancer development and therapy. Cancers. 2021;13(8):1946.
Chen S, Li J, Meng S, He T, Shi Z, Wang C, et al. Microglia and macrophages in the neuro-glia-vascular unit: from identity to functions. Neurobiol Dis. 2023;179:106066.
Christie JD, Chiocca EA. Treat and repeat: oncolytic virus therapy for brain cancer. Nat Med. 2022;28(8):1540–2.
Cui X, Morales RT, Qian W, Wang H, Gagner JP, Dolgalev I, et al. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials. 2018;161:164–78.
De Boeck A, Ahn BY, D’Mello C, Lun X, Menon SV, Alshehri MM, et al. Glioma-derived IL-33 orchestrates an inflammatory brain tumor microenvironment that accelerates glioma progression. Nat Commun. 2020;11(1):4997.
De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–74.
Di Nunno V, Aprile M, Gatto L, Tosoni A, Ranieri L, Bartolini S, et al. Tumor Microenvironment in Gliomas: a treatment hurdle or an opportunity to grab? Cancers (Basel). 2023;15(4):1042.
Ding P, Wang W, Wang J, Yang Z, Xue L. Expression of tumor-associated macrophage in progression of human glioma. Cell Biochem Biophys. 2014;70(3):1625–31.
Dong F, Qin X, Wang B, Li Q, Hu J, Cheng X, et al. ALKBH5 facilitates Hypoxia-induced paraspeckle assembly and IL8 secretion to generate an immunosuppressive tumor microenvironment. Cancer Res. 2021;81(23):5876–88.
Fields GB. Mechanisms of action of novel drugs targeting angiogenesis-promoting Matrix metalloproteinases. Front Immunol. 2019;10:1278.
Fierro J Jr., DiPasquale J, Perez J, Chin B, Chokpapone Y, Tran AM, et al. Dual-sgRNA CRISPR/Cas9 knockout of PD-L1 in human U87 glioblastoma tumor cells inhibits proliferation, invasion, and tumor-associated macrophage polarization. Sci Rep. 2022;12(1):2417.
Flores-Toro JA, Luo D, Gopinath A, Sarkisian MR, Campbell JJ, Charo IF, et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc Natl Acad Sci. 2020;117(2):1129–38.
Foray C, Barca C, Winkeler A, Wagner S, Hermann S, Schäfers M, et al. Interrogating Glioma-associated Microglia and Macrophage dynamics under CSF-1R therapy with multitracer in vivo PET/MRI. J Nucl Med. 2022;63(9):1386–93.
Fujita Y, Yamashita T. Mechanisms and significance of microglia-axon interactions in physiological and pathophysiological conditions. Cell Mol Life Sci. 2021;78(8):3907–19.
Gangoso E, Southgate B, Bradley L, Rus S, Galvez-Cancino F, McGivern N, et al. Glioblastomas acquire myeloid-affiliated transcriptional programs via epigenetic immunoediting to elicit immune evasion. Cell. 2021;184(9):2454–e24702426.
Geraldo LH, Xu Y, Jacob L, Pibouin-Fragner L, Rao R, Maissa N, et al. SLIT2/ROBO signaling in tumor-associated microglia and macrophages drives glioblastoma immunosuppression and vascular dysmorphia. J Clin Invest. 2021;131(16)141083.
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708.
Gilbert MR, Pugh SL, Aldape K, Sorensen AG, Mikkelsen T, Penas-Prado M, et al. NRG oncology RTOG 0625: a randomized phase II trial of bevacizumab with either irinotecan or dose-dense temozolomide in recurrent glioblastoma. J Neurooncol. 2017;131(1):193–9.
Goswami S, Walle T, Cornish AE, Basu S, Anandhan S, Fernandez I, et al. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat Med. 2020;26(1):39–46.
Guilliams M, Thierry GR, Bonnardel J, Bajenoff M. Establishment and maintenance of the Macrophage Niche. Immunity. 2020;52(3):434–51.
Guo Y, Hong W, Wang X, Zhang P, Körner H, Tu J, et al. MicroRNAs in Microglia: how do MicroRNAs affect activation, inflammation, polarization of Microglia and mediate the Interaction between Microglia and Glioma? Front Mol Neurosci. 2019;12:125.
Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–7.
Hara T, Chanoch-Myers R, Mathewson ND, Myskiw C, Atta L, Bussema L, et al. Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma. Cancer Cell. 2021;39(6):779–e792711.
Hasan MN, Luo L, Ding D, Song S, Bhuiyan MIH, Liu R, et al. Blocking NHE1 stimulates glioma tumor immunity by restoring OXPHOS function of myeloid cells. Theranostics. 2021;11(3):1295–309.
He C, Sheng L, Pan D, Jiang S, Ding L, Ma X, et al. Single-cell transcriptomic analysis revealed a critical role of SPP1/CD44-Mediated crosstalk between macrophages and cancer cells in Glioma. Front Cell Dev Biol. 2021;9:779319.
Hu J, Dong F, He Y, Xia X, Cheng F, Chen S, et al. LRIG2 promotes glioblastoma progression by modulating innate antitumor immunity through macrophage infiltration and polarization. J Immunother Cancer. 2022;10(9):e004452.
Huang B, Luo L, Wang J, He B, Feng R, Xian N, et al. B7-H3 specific T cells with chimeric antigen receptor and decoy PD-1 receptors eradicate established solid human tumors in mouse models. Oncoimmunology. 2020;9(1):1684127.
Jiang N, Xie B, Xiao W, Fan M, Xu S, Duan Y, et al. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat Commun. 2022;13(1):1511.
Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, et al. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39(1):204.
Khan F, Pang L, Dunterman M, Lesniak MS, Heimberger AB, Chen P. Macrophages and microglia in glioblastoma: heterogeneity, plasticity, and therapy. J Clin Invest. 2023;133(1):e163446.
Kierdorf K, Masuda T, Jordão MJC, Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci. 2019;20(9):547–62.
Kierdorf K, Prinz M. Microglia in steady state. J Clin Invest. 2017;127(9):3201–9.
Kim HJ, Park JH, Kim HC, Kim CW, Kang I, Lee HK. Blood monocyte-derived CD169(+) macrophages contribute to antitumor immunity against glioblastoma. Nat Commun. 2022;13(1):6211.
Kirstein A, Schmid TE, Combs SE. The role of miRNA for the treatment of MGMT unmethylated Glioblastoma multiforme. Cancers (Basel). 2020;12(5):1099.
Kiyokawa J, Kawamura Y, Ghouse SM, Acar S, Barçın E, Martínez-Quintanilla J, et al. Modification of extracellular matrix enhances oncolytic adenovirus immunotherapy in Glioblastoma. Clin Cancer Res. 2021;27(3):889–902.
Knudsen AM, Halle B, Cédile O, Burton M, Baun C, Thisgaard H, et al. Surgical resection of glioblastomas induces pleiotrophin-mediated self-renewal of glioblastoma stem cells in recurrent tumors. Neuro Oncol. 2022;24(7):1074–87.
Kumar A, Mohamed E, Tong S, Chen K, Mukherjee J, Lim Y, et al. CXCL14 promotes a robust brain tumor-associated immune response in Glioma. Clin Cancer Res. 2022;28(13):2898–910.
Lamano JB, Lamano JB, Li YD, DiDomenico JD, Choy W, Veliceasa D, et al. Glioblastoma-derived IL6 induces immunosuppressive peripheral myeloid cell PD-L1 and promotes Tumor Growth. Clin Cancer Res. 2019;25(12):3643–57.
Lanza M, Casili G, Campolo M, Paterniti I, Colarossi C, Mare M, et al. Immunomodulatory effect of microglia-released cytokines in Gliomas. Brain Sci. 2021;11(4):466.
Leavitt RJ, Acharya MM, Baulch JE, Limoli CL. Extracellular vesicle-derived miR-124 resolves radiation-induced brain injury. Cancer Res. 2020;80(19):4266–2477.
Lee AH, Sun L, Mochizuki AY, Reynoso JG, Orpilla J, Chow F, et al. Neoadjuvant PD-1 blockade induces T cell and cDC1 activation but fails to overcome the immunosuppressive tumor associated macrophages in recurrent glioblastoma. Nat Commun. 2021;12(1):6938.
Levin M, Udi Y, Solomonov I, Sagi I. Next generation matrix metalloproteinase inhibitors novel strategies bring new prospects. Biochimica et Biophysica Acta (BBA) - Mol Cell Res. 2017;1864(11):1927–39.
Li N, Qin J, Lan L, Zhang H, Liu F, Wu Z, et al. PTEN inhibits macrophage polarization from M1 to M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer Biol Ther. 2015;16(2):297–306.
Li HY, McSharry M, Bullock B, Nguyen TT, Kwak J, Poczobutt JM, et al. The tumor microenvironment regulates sensitivity of murine lung tumors to PD-1/PD-L1 antibody blockade. Cancer Immunol Res. 2017;5(9):767–77.
Li Z, Zhang J, Zheng H, Li C, Xiong J, Wang W, et al. Modulating lncRNA SNHG15/CDK6/miR-627 circuit by palbociclib, overcomes temozolomide resistance and reduces M2-polarization of glioma associated microglia in glioblastoma multiforme. J Exp Clin Cancer Res. 2019;38(1):380.
Li M, Kirtane AR, Kiyokawa J, Nagashima H, Lopes A, Tirmizi ZA, et al. Local targeting of NAD(+) salvage pathway alters the immune tumor microenvironment and enhances checkpoint immunotherapy in Glioblastoma. Cancer Res. 2020;80(22):5024–34.
Li J, Kaneda MM, Ma J, Li M, Shepard RM, Patel K,et al. PI3Kγ inhibition suppresses microglia/TAM accumulation in glioblastoma microenvironment to promote exceptional temozolomide response. ProcNatl Acad Sci U S A. 2021;118(16):e2009290118.
Li M, He L, Zhu J, Zhang P, Liang S. Targeting tumor-associated macrophages for cancer treatment. Cell Bioscience. 2022a;12(1):85.
Li B, Yang C, Zhu Z, Chen H, Qi B. Hypoxic glioma-derived extracellular vesicles harboring microRNA-10b-5p enhance M2 polarization of macrophages to promote the development of glioma. CNS Neurosci Ther. 2022b;28(11):1733–47.
Li M, Xu H, Qi Y, Pan Z, Li B, Gao Z, et al. Tumor-derived exosomes deliver the tumor suppressor mir-3591-3p to induce M2 macrophage polarization and promote glioma progression. Oncogene. 2022c;41(41):4618–32.
Li D, Zhang Q, Li L, Chen K, Yang J, Dixit D, et al. β2-Microglobulin maintains Glioblastoma stem cells and induces M2-like polarization of tumor-associated macrophages. Cancer Res. 2022;82(18):3321–34.
Li G, Li L, Li Y, Qian Z, Wu F, He Y, et al. An MRI radiomics approach to predict survival and tumour-infiltrating macrophages in gliomas. Brain. 2022e;145(3):1151–61.
Li Z, Fu WJ, Chen XQ, Wang S, Deng RS, Tang XP, et al. Autophagy-based unconventional secretion of HMGB1 in glioblastoma promotes chemosensitivity to temozolomide through macrophage M1-like polarization. J Exp Clin Cancer Res. 2022f;41(1):74.
Li S, Chen J, Fan Y, Wang C, Wang C, Zheng X, et al. Liposomal honokiol induces ROS-mediated apoptosis via regulation of ERK/p38-MAPK signaling and autophagic inhibition in human medulloblastoma. Signal Transduct Target Ther. 2022g;7(1):49.
Lin E, Liu X, Liu Y, Zhang Z, Xie L, Tian K, et al. Roles of the dynamic tumor immune microenvironment in the individualized treatment of advanced clear cell renal cell carcinoma. Front Immunol. 2021;12;653358.
Liu T, Zhu C, Chen X, Guan G, Zou C, Shen S, et al. Ferroptosis, as the most enriched programmed cell death process in glioma, induces immunosuppression and immunotherapy resistance. Neuro Oncol. 2022;24(7):1113–25.
Liu X, Li Z, Sun J, Zhang Z, Li W. Interaction between PD-L1 and soluble VEGFR1 in glioblastoma-educated macrophages. BMC Cancer. 2023;23(1):259.
Lu J, Xu Z, Duan H, Ji H, Zhen Z, Li B, et al. Tumor-associated macrophage interleukin-β promotes glycerol-3-phosphate dehydrogenase activation, glycolysis and tumorigenesis in glioma cells. Cancer Sci. 2020;111(6):1979–90.
Ma W, Zhang K, Bao Z, Jiang T, Zhang Y. SAMD9 is relating with M2 macrophage and remarkable malignancy characters in low-Grade Glioma. Front Immunol. 2021;12:659659.
Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discovery. 2022;21(11):799–820.
Mass E, Nimmerjahn F, Kierdorf K, Schlitzer A. Tissue-specific macrophages: how they develop and choreograph tissue biology. Nat Rev Immunol. 2023;23:563.
Mohme M, Neidert MC. Tumor-specific T cell activation in malignant brain tumors. Front Immunol. 2020;11:205.
Müller Bark J, Kulasinghe A, Chua B, Day BW, Punyadeera C. Circulating biomarkers in patients with glioblastoma. Br J Cancer. 2020;122(3):295–305.
Mundt S, Greter M, Becher B. The CNS mononuclear phagocyte system in health and disease. Neuron. 2022;110(21):3497–512.
Muzio L, Viotti A, Martino G. Microglia in Neuroinflammation and Neurodegeneration: from understanding to therapy. Front Neurosci. 2021;15:742065.
Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, et al. An Integrative Model of Cellular States, plasticity, and Genetics for Glioblastoma. Cell. 2019;178(4):835–e849821.
Pan Z, Zhao R, Li B, Qi Y, Qiu W, Guo Q, et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol Cancer. 2022;21(1):16.
Park MD, Silvin A, Ginhoux F, Merad M. Macrophages in health and disease. Cell. 2022;185(23):4259–79.
Peng Y, Chen F, Li S, Liu X, Wang C, Yu C, et al. Tumor-associated macrophages as treatment targets in glioma. Brain Sci Adv. 2020;6(4):306–23.
Peng P, Zhu H, Liu D, Chen Z, Zhang X, Guo Z, et al. TGFBI secreted by tumor-associated macrophages promotes glioblastoma stem cell-driven tumor growth via integrin αvβ5-Src-Stat3 signaling. Theranostics. 2022;12(9):4221–36.
Pietras A, Katz AM, Ekström EJ, Wee B, Halliday JJ, Pitter KL, et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14(3):357–69.
Pijuan J, Barceló C, Moreno DF, Maiques O, Sisó P, Marti RM, et al. In vitro cell migration, invasion, and adhesion assays: from cell imaging to data analysis. Front Cell Dev Biol. 2019;7:107.
Pombo Antunes AR, Scheyltjens I, Lodi F, Messiaen J, Antoranz A, Duerinck J, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci. 2021;24(4):595–610.
Prosniak M, Harshyne LA, Andrews DW, Kenyon LC, Bedelbaeva K, Apanasovich TV, et al. Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res. 2013;19(14):3776–86.
Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–72.
Qian M, Wang S, Guo X, Wang J, Zhang Z, Qiu W, et al. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-κB pathways. Oncogene. 2020;39(2):428–42.
Quintero-Fabián S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V, Lara-Riegos J, et al. Role of matrix Metalloproteinases in Angiogenesis and cancer. FrontOncol. 2019;9:1370.
Rao R, Han R, Ogurek S, Xue C, Wu LM, Zhang L, et al. Glioblastoma genetic drivers dictate the function of tumor-associated macrophages/microglia and responses to CSF1R inhibition. Neuro Oncol. 2022;24(4):584–97.
Romano G, Nigita G, Calore F, Saviana M, Le P, Croce CM, et al. MiR-124a regulates extracellular vesicle release by targeting GTPase rabs in lung cancer. Front Oncol. 2020;10:1454.
Ronaldson PT, Davis TP. Regulation of blood-brain barrier integrity by microglia in health and disease: a therapeutic opportunity. J Cereb Blood Flow Metab. 2020;40(1suppl):S6–24.
Ruan J, Ouyang M, Zhang W, Luo Y, Zhou D. The effect of PD-1 expression on tumor-associated macrophage in T cell lymphoma. Clin Transl Oncol. 2021;23(6):1134–41.
Saha D, Martuza RL, Rabkin SD. Macrophage polarization contributes to Glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32(2):253–e267255.
Sahin E, Haubenwallner S, Kuttke M, Kollmann I, Halfmann A, Dohnal AM, et al. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J Immunol. 2014;193(4):1717–27.
Sanchez Gil J, Rabkin SD. An armed oncolytic virus for GBM destruction. Nat Cancer. 2022;3(11):1274–6.
Shi Y, Zhang B, Zhu J, Huang W, Han B, Wang Q, et al. MiR-106b-5p inhibits IRF1/IFN-β signaling to promote M2 macrophage polarization of Glioblastoma. Onco Targets Ther. 2020;13:7479–92.
Takenaka MC, Gabriely G, Rothhammer V, Mascanfroni ID, Wheeler MA, Chao CC, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci. 2019;22(5):729–40.
Tan Y, Wang M, Zhang Y, Ge S, Zhong F, Xia G, et al. Tumor-associated macrophages: a potential target for cancer therapy. Front Oncol. 2021;11:693517.
Tan IL, Arifa RDN, Rallapalli H, Kana V, Lao Z, Sanghrajka RM, et al. CSF1R inhibition depletes tumor-associated macrophages and attenuates tumor progression in a mouse sonic hedgehog-medulloblastoma model. Oncogene. 2021b;40(2):396–407.
Tang X, Zhao S, Zhang Y, Wang Y, Zhang Z, Yang M, et al. B7-H3 as a novel CAR-T therapeutic target for Glioblastoma. Mol Ther Oncolytics. 2019;14:279–87.
Tao W, Chu C, Zhou W, Huang Z, Zhai K, Fang X, et al. Dual role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. Nat Commun. 2020;11(1):3015.
Tong N, He Z, Ma Y, Wang Z, Huang Z, Cao H, et al. Tumor Associated macrophages, as the Dominant Immune cells, are an indispensable target for immunologically cold tumor-glioma therapy? Front Cell Dev Biol. 2021;9:706286.
Tu MM, Abdel-Hafiz HA, Jones RT, Jean A, Hoff KJ, Duex JE, et al. Inhibition of the CCL2 receptor, CCR2, enhances tumor response to immune checkpoint therapy. Commun Biology. 2020;3(1):720.
Tu J, Fang Y, Han D, Tan X, Xu Z, Jiang H, et al. MicroRNA-22 represses glioma development via activation of macrophage-mediated innate and adaptive immune responses. Oncogene. 2022;41(17):2444–57.
Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, et al. Tumor evolution of Glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32(1):42–e5646.
Wang J, Leavenworth JW, Hjelmeland AB, Smith R, Patel N, Borg B, et al. Deletion of the RNA regulator HuR in tumor-associated microglia and macrophages stimulates anti-tumor immunity and attenuates glioma growth. Glia. 2019;67(12):2424–39.
Wang QW, Sun LH, Zhang Y, Wang Z, Zhao Z, Wang ZL,et al. MET overexpression contributes to STAT4-PD-L1 signaling activation associated with tumor-associated, macrophages-mediated immunosuppression in primary glioblastomas. J Immunother Cancer. 2021;9(10):e002451.
Wang X, Ding H, Li Z, Peng Y, Tan H, Wang C, et al. Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects. Signal Transduct Target Ther. 2022;7(1):74.
Wculek SK, Dunphy G, Heras-Murillo I, Mastrangelo A, Sancho D. Metabolism of tissue macrophages in homeostasis and pathology. Cell Mol Immunol. 2022;19(3):384–408.
Wei J, Marisetty A, Schrand B, Gabrusiewicz K, Hashimoto Y, Ott M, et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest. 2019;129(1):137–49.
Wei J, Chen P, Gupta P, Ott M, Zamler D, Kassab C, et al. Immune biology of glioma-associated macrophages and microglia: functional and therapeutic implications. Neuro Oncol. 2020;22(2):180–94.
Wei Q, Singh O, Ekinci C, Gill J, Li M, Mamatjan Y, et al. TNFα secreted by glioma associated macrophages promotes endothelial activation and resistance against anti-angiogenic therapy. Acta Neuropathol Commun. 2021;9(1):67.
Wei C, Wang B, Peng D, Zhang X, Li Z, Luo L, et al. Pan-cancer Analysis shows that ALKBH5 is a potential prognostic and immunotherapeutic biomarker for multiple cancer types including gliomas. Front Immunol. 2022;13:849592.
Wu H, Liu J, Wang Z, Yuan W, Chen L. Prospects of antibodies targeting CD47 or CD24 in the treatment of glioblastoma. CNS Neurosci Ther. 2021;27(10):1105–17.
Xiao Y, Wang Z, Zhao M, Deng Y, Yang M, Su G, et al. Single-cell transcriptomics revealed subtype-specific tumor immune microenvironments in human glioblastomas. Front Immunol. 2022;13:914236.
Xu J, Zhang J, Zhang Z, Gao Z, Qi Y, Qiu W, et al. Hypoxic glioma-derived exosomes promote M2-like macrophage polarization by enhancing autophagy induction. Cell Death Dis. 2021;12(4):373.
Xu Z, Chen X, Song L, Yuan F, Yan Y. Matrix Remodeling-Associated protein 8 as a Novel Indicator contributing to Glioma Immune response by regulating Ferroptosis. Front Immunol. 2022a;13:834595.
Xu J, Zheng Y, Wang L, Liu Y, Wang X, Li Y, et al. MiR-124: a promising therapeutic target for central nervous system injuries and diseases. Cell Mol Neurobiol. 2022;42(7):2031–53.
Xuan W, Lesniak MS, James CD, Heimberger AB, Chen P. Context-dependent Glioblastoma-Macrophage/Microglia symbiosis and associated mechanisms. Trends Immunol. 2021;42(4):280–92.
Xue N, Zhou Q, Ji M, Jin J, Lai F, Chen J, et al. Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci Rep. 2017;7:39011.
Yang F, He Z, Duan H, Zhang D, Li J, Yang H, et al. Synergistic immunotherapy of glioblastoma by dual targeting of IL-6 and CD40. Nat Commun. 2021;12(1):3424.
Yang T, Hu Y, Miao J, Chen J, Liu J, Cheng Y, et al. A BRD4 PROTAC nanodrug for glioma therapy via the intervention of tumor cells proliferation, apoptosis and M2 macrophages polarization. Acta Pharm Sin B. 2022a;12(6):2658–71.
Yang K, Han W, Jiang X, Piffko A, Bugno J, Han C, et al. Zinc cyclic di-AMP nanoparticles target and suppress tumours via endothelial STING activation and tumour-associated macrophage reinvigoration. Nat Nanotechnol. 2022b;17(12):1322–31.
Yin J, Valin KL, Dixon ML, Leavenworth JW. The role of Microglia and macrophages in CNS Homeostasis, autoimmunity, and Cancer. J Immunol Res. 2017;2017:5150678.
Yin J, Kim SS, Choi E, Oh YT, Lin W, Kim TH, et al. ARS2/MAGL signaling in glioblastoma stem cells promotes self-renewal and M2-like polarization of tumor-associated macrophages. Nat Commun. 2020;11(1):2978.
Yin W, Ping YF, Li F, Lv SQ, Zhang XN, Li XG, et al. A map of the spatial distribution and tumour-associated macrophage states in glioblastoma and grade 4 IDH-mutant astrocytoma. J Pathol. 2022;258(2):121–35.
Yu M, Chang Y, Zhai Y, Pang B, Wang P, Li G, et al. TREM2 is associated with tumor immunity and implies poor prognosis in glioma. Front Immunol. 2022;13:1089266.
Yu-Ju Wu C, Chen CH, Lin CY, Feng LY, Lin YC, et al. CCL5 of glioma-associated microglia/macrophages regulates glioma migration and invasion via calcium-dependent matrix metalloproteinase 2. Neuro Oncol. 2020;22(2):253–66.
Zeiner PS, Preusse C, Golebiewska A, Zinke J, Iriondo A, Muller A, et al. Distribution and prognostic impact of microglia/macrophage subpopulations in gliomas. Brain Pathol. 2019;29(4):513–29.
Zhai K, Huang Z, Huang Q, Tao W, Fang X, Zhang A, et al. Pharmacological inhibition of BACE1 suppresses glioblastoma growth by stimulating macrophage phagocytosis of tumor cells. Nat Cancer. 2021;2(11):1136–51.
Zhang Y, Yu G, Chu H, Wang X, Xiong L, Cai G, et al. Macrophage-Associated PGK1 phosphorylation promotes aerobic glycolysis and Tumorigenesis. Mol Cell. 2018;71(2):201–e215207.
Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun. 2019;10(1):3974.
Zhang S-Y, Song X-Y, Li Y, Ye L-L, Zhou Q, Yang W-B. Tumor-associated macrophages: a promising target for a cancer immunotherapeutic strategy. Pharmacol Res. 2020;161:105111.
Zhang G, Tao X, Ji B, Gong J. Hypoxia-driven M2-polarized macrophages facilitate cancer aggressiveness and Temozolomide resistance in Glioblastoma. OxidMed Cell Longev. 2022;2022:1614336.
Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther. 2020;5(1):201.
Zheng Z, Zhang J, Jiang J, He Y, Zhang W, Mo X, et al. Remodeling tumor immune microenvironment (TIME) for glioma therapy using multi-targeting liposomal codelivery. J Immunother Cancer. 2020;8(2):e000207.
Zhou LJ, Peng J, Xu YN, Zeng WJ, Zhang J, Wei X, et al. Microglia are indispensable for synaptic plasticity in the spinal dorsal horn and chronic pain. Cell Rep. 2019;27(13):3844–e38593846.
Zhou F, Shi Q, Fan X, Yu R, Wu Z, Wang B, et al. Diverse macrophages constituted the Glioma microenvironment and influenced by PTEN status. Front Immunol. 2022;13:841404.
Zhou Y, Meng X, He W, Li X, Zhao R, Dong C, et al. USF1/CD90 signaling in maintaining glioblastoma stem cells and tumor-associated macrophages adhesion. Neuro Oncol. 2022b;24(9):1482–93.
Zhu Z, Zhang H, Chen B, Liu X, Zhang S, Zong Z, et al. PD-L1-Mediated immunosuppression in Glioblastoma is associated with the infiltration and M2-polarization of tumor-associated macrophages. Front Immunol. 2020;11:588552.
Zhu Y, Liang J, Gao C, Wang A, Xia J, Hong C, et al. Multifunctional ginsenoside Rg3-based liposomes for glioma targeting therapy. J Contr Rel. 2021;330:641–57.
Funding
This article was supported by the Beijing Clinical Key Specialty Project (2-1-2-038) and the Training Fund for Open Projects at Clinical Institutes and Departments of Capital Medical University.
Author information
Authors and Affiliations
Contributions
Jiachen Wang drafted the initial manuscript and created the original figures. Shenglan Li critically reviewed the intellectual content of the manuscript and made substantial revisions. Yanjie Lan and Xinrui Liu critically reviewed the intellectual content of the manuscript. Wenbin Li designed the study and was responsible for it. He also critically reviewed the intellectual content of the manuscript and obtained funding for the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This manuscript does not report on or involve any animals, humans, human data, human tissue or plants, so no ethical approval and consent to publish were required.
Consent for publication
This manuscript contains original images, which have been obtained with the consent of the original author. The author has given permission to use the images for the purpose of this manuscript and has agreed to publish them. The author has also confirmed that the images do not infringe any third-party rights or privacy.
Competing interests
Author Wenbin Li is a member of the Editorial Board for Current Medicine. The paper was handled by the other Editor and has undergone rigorous peer review process. Author wenbin Li was not involved in the journal’s review of, or decisions related to, this manuscript.
The other authors of this manuscript declare that they have no competing interests related to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., Li, S., Lan, Y. et al. Glioma-associated macrophages: unraveling their dual role in the microenvironment and therapeutic implications. Curr Med 3, 4 (2024). https://doi.org/10.1007/s44194-024-00031-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44194-024-00031-y