Abstract
Primary central nervous system lymphoma is a kind of extranodal lymphoma with high degree of malignancy, hidden onset and strong invasion. It is a special type of non-Hodgkin’s lymphoma and very rare in clinic. Due to little understanding of the pathogenesis and high risk factors of the disease, there are great differences in the prevention, staging and treatment plan of the disease, and there is no strict standard. In this review, we aim to comprehensively summarize the clinical characteristics of PCNSL and the promising clinical treatment strategies for PCNSL to date.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
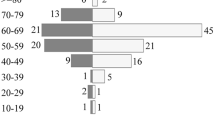
Primary central nervous system lymphoma (PCNSL) refers to a rare and highly invasive extra-nodal non-Hodgkin lymphoma limited to the brain, pia meninges, eyes, and spinal cord without systemic invasion, accounting for 3-4% of all primary brain tumors and 4-6% of extra-nodal lymphomas [1]. But some studies suggest its incidence is increasing [2, 3]. A summary of PCNSL data from nationwide population-based studies in Western Europe, North America, and Asia found an annual incidence of between 0.3 and 0.5 per 100,000 people. Based on the Surveillance, Epidemiology, and End Results (SEER) Registry research database, the incidence rate in the United States continued to increase by a factor of five from 1975 to 2017, consistent with other countries. However, the incidence of PCNSL varies greatly by age group, and patients ≥ 60 years old are the most increasing group and account for the majority of patients, and the median age of patients is 68 years old [4]. Before 2000, the improvement in survival of other lymphomas in China was much lower than that in developed countries abroad. However, for central nervous system lymphoma, both at home and abroad, the survival prognosis is poor, and the 3-year survival rate is only 30%.With the development of emerging drugs and therapies, the 5-year survival rate of patients with PCNSL has increased and the mortality rate has decreased, and the impact on the prognosis of patients is also very obvious. But challenges remain and require further research and investigation.
2 Patholog
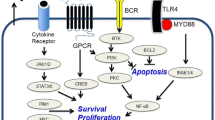
About 90% of PCNSLs are diffuse large B-cell lymphomas (DLBCLs), and the remainder are T-cell lymphomas, low-grade lymphomas, or Burkitt lymphomas [5, 6]. Most PCNSLS express pan-B-cell markers, The vast majority of highly expressed B-cell lymphomas 6-BCL-6 (60–80%) and multiple myeloma oncogene 1/ interferon regulatory factor 4-MUM1 /IRF4(90%). Tumor cells were highly proliferative, with > 90% expressing the proliferation-associated Ki67 antigen [7]. Genomic analysis of PCNSL showed that MYD88 and CD79B mutations were very common in over 90% of cases, CARD11 and TNFAIP3 mutations were rare, and other genetic alterations were consistent with activation of B-cell receptor, Toll-like receptor, and nuclear factor-KB pathways. Other genes mutated in PCNSL included PIM1(Pim-1 proto-oncogene, serine/threonine kinase), BTG2(B-cell translocation gene 2), PRDMI(PR domain zinc finger protein 1), TOX(Thymocyte Selection Associated High Mobility Group Box), and IRF4(Interferon regulatory factor 4, IRF4). CDKN2A inactivation was also noted. These pathological features provide researchers with useful ideas for clinical study design [8,9,10].
From then on, With the progress of various assistive technologies, people have become more detailed and specific about the genotyping of DLBCL. At the same time, detailed genotyping has laid a solid foundation for the advent of the era of precision therapy of DLBCL. However, it is unclear whether the genotypes of primary CNS DLBCL and peripheral DLBCL are consistent. According to whether the primary central nervous system DLBCL patients carry CD79B and PIM1 mutations, the team of Professor Huang Yuhua, and Professor Zhang Xinyou, Shenzhen People’s Hospital, divided the patients into CDP group and non-CDP group. The 2-year OS rate was found to be higher in CDP patients than in non-CDP patients (76% vs. 40%) [11]. However, the results of this study need to further clarify whether primary CNS DLBCL can be further categorized like peripheral DLBCL, which may be the key to unlocking the precision therapy of primary CNS DLBCL. However, it is unclear whether the genotypes of primary CNS DLBCL and peripheral DLBCL are consistent. According to whether the primary central nervous system DLBCL patients carry CD79B and PIM1 mutations, the team of Professor Huang Yuhua, and Professor Zhang Xinyou, Shenzhen People’s Hospital, divided the patients into CDP group and non-CDP group. The 2-year OS rate was found to be higher in CDP patients than in non-CDP patients (76% vs. 40%). However, the results of this study need to further clarify whether primary CNS DLBCL can be further categorized like peripheral DLBCL, which may be the key to unlocking the precision therapy of primary CNS DLBCL.
3 Clinical manifestations and diagnosis
The clinical manifestations of PCNSL patients are complex and varied according to different sites of central nervous system lesions. Patients may present with focal nerve defects, neuropsychiatric signs, symptoms of elevated intracranial pressure, or ocular symptoms. Symptoms of eye involvement include blurred vision and floaters [12,13,14]. Unlike patients with generalized NHL, patients with PCNSL rarely experience symptoms of B, such as fever, night sweats, or weight loss. Currently, PCNSL is diagnosed by stereotactic biopsy or immunocytological labeling of cerebrospinal fluid material or flow cytometry [15]. There have been numerous studies on prognostic factors of PCNSL. Currently, the comprehensive prognostic assessment is mainly conducted using the prognostic scores recommended by the International Extranodal Lymphoma Working Group (IELSG) and MSKCC. The specific parameters of the IELSG scoring system are detailed in Table 1. The groups of patients are shown in Table 2.The detailed grouping of MSKCC prognostic model is shown in Table 3. Based on these three classifications, significant differences in overall survival and progression-free survival were observed between the two scoring systems [16, 17].
Due to its difficult early diagnosis, the prognosis for PCNSL that has failed first-line treatment remains poor. While novel and effective treatments have improved survival rates, the management of the disease remains a tough and thorny issues in oncology.
4 Treatment of newly diagnosed PCNSL
On account of the multifocal, deep, and aggressive nature of PCNSL, excision is not part of the standard treatment, surgical excision of the lesion; Delay chemotherapy and cause surgery-related complications. Brain biopsy is preferred. Excision is not recommended unless it contributes to neurological symptoms and mass effects [18, 19]. Treatment of newly diagnosed PCNSL includes induction and consolidation phases. Induction therapy involves chemotherapy and aims to achieve complete remission. Large dose of methotrexate (3.5 g/m2 and above) can effectively pass the blood-brain barrier, and is considered the cornerstone of the treatment of PCNSL and by far the most effective drug, it is often the first choice of clinicians. The role of rituximab (the standard drug for systemic DLBCL chemotherapy) as part of hd MTX-based induced comprehensive chemotherapy is controversial because it has produced paradoxical effects in different clinical trials: the Phase II study of IELSG 32 showed an overall benefit for patients using the drug [20, 21], however, the HOVON 105/ALLG NHL 24 Phase III study, which was designed to address this issue, showed that patients as a whole did not benefit from the drug [22]. A meta-analysis of two randomized studies found that rituximab was beneficial for PFS (but the scientific and accuracy is low, can not be used as clinical guidance evidence), but not statistically significant for OS [23]. So in practice, multiple induction chemotherapy regimens can be used in combination with hd MTX and other drugs, Such as R-MPVA (rituximab, hd MTX, Procarbazide, vincristine, cytarabine), MATRIX protocol (hd MTX, Cytarabine, Cetepi, rituximab), MBVP (Cytarabine, VP16, carmostine, prednisolone), MTR (hd MTX, Temozolomide and rituximab) are among the most recognized drugs, and the complete response rate of different induction chemotherapy regimens is 40–66%. In the near future, our efforts in induction chemotherapy regiments can be further improved by adding neytype drugs such as immunomodulators, BTK, immune checkpoint inhibitors, etc. These drugs have shown considerable efficacy as monotherapy in the clinical treatment of recurrent PCNSL [24].
The purpose of consolidation is to prevent recurrence of the disease. Multiple consolidation therapies are currently being used, with the primary purpose of consolidation being to significantly improve progression-free survival and delay recurrence. For patients who obtain CR or CRu after induction chemotherapy, autologous hematopoietic stem cell transplantation or total craniocerebral radiotherapy can be used for consolidation therapy [25].
Historically, PCNSL has only been treated with WBRT at doses of 36–45 Gy, which has resulted in a higher percentage of radiation reactions, but also early recurrence in less than a year. Because patients do not consistently benefit from radiation therapy and there are significant neurotoxic side effects that affect quality of life, WBRT has not been prioritized in the initial treatment of patients, but can be used in subsequent patient consolidation or salvage treatment. Intraocular injection or ocular involvement field radiotherapy may be performed in patients with no remission of ocular lesions after induction chemotherapy [26]. The RTOG 1114 randomized Phase II study evaluated the effect of reduced doses of WBRT, and preliminary data indicated that the addition of low-dose WBRT (23.4 Gy, 1.8 Gy fractional) to immunochemotherapy induction (R-MPV-A) improved PFS in newly diagnosed PCNSL compared with immunochemotherapy alone [27]. At the time of analysis, there was no statistically significant increase in neurotoxicity assessed by neuropsychological tests, but this does not mean that we need to do nothing and need to establish longer communication with patients, especially for older patients, who are more prone to unexpected long-term adverse effects.
The BLOCAGE Phase III study, specifically for older patients, is evaluating the role of maintenance therapy (MTR) in patients with complete response after hd MTX-based induction chemotherapy (NCT02313389). The ALLIANCE A51901 Phase I study is studying dose-increasing lenalidomide in combination with hd MTX and rituximab (with or without natuliumab) followed by low-dose lenalidomide maintenance therapy in elderly patients with newly diagnosed PCNSL who are not candidates for autologous stem cell transplantation (NCT04609046). The FIORELLA study randomly assigned patients to procarbazine or lenalidomide as maintenance therapy after a combination of MTX, procarbazine, and rituximab (NCT03495960). Elderly patients who are not suitable for hd MTX therapy due to renal insufficiency or poor condition, and who are at higher risk of developing systemic toxicity, may receive well-tolerated treatment in maintenance regiments.
5 Treatment of Refractory and Relapsed(R/R) PCNSL
Although the initial treatment based on hd-mtx results in a majority of patients achieving remission, more than half of patients relapse, with the first two years being the peak of relapse. The prognosis for R/R PCNSL is poor, and although relapse of PCNSL occurs primarily within the central nervous system, up to 17% of patients have been reported to occur in extraneural organs [28]. Late relapses of primary CNS DLBCL appear to be more common than systemic DLBCL. Subsequent treatment options can be determined based on the initial treatment regimen and time to relapse, but no optimal regimen is recommended [29]. If the first-line methotrexate regimen is used and the efficacy is maintained for more than 1 year, high-dose methotrexate can be used again. For early recurrence, conversion to full-frequency brain radiotherapy or other second-line regiments is recommended. Without better systemic treatment, WBRT is an option for many patients who are faced with the dilemma of multiple disease relapses or rapid uncontrolled disease progression, but the serious neurocognitive complications of this treatment must be taken into account when deciding on this treatment option. Autologous hematopoietic thousand cell transplantation may also be used as a consolidation therapy if remission is achieved. Immunotherapy, signaling pathway blocking and other therapies were used to rescue the recurrent and refractory PCNSL [30].
6 Novel approaches and targeted therapies
Overall, for central nervous system lymphoma, survival has been greatly improved in recent years with the introduction of several new drugs and the development of precision diagnosis and treatment. New therapies currently being investigated for primary central nervous system DLBCL include BTK inhibitors, IMiDs, mTOR inhibitors, pI3K inhibitors, immune checkpoint inhibitors, and CD19-directed CAR T cells, and have entered clinical trials [8].
After constant exploration, a number of studies have found that MYD88 and other sudden interactions exist at a high frequency in PCNSL. In promising personalized therapy, inhibitors targeting BTK, the signaling mediator of BCR, show a good effect on relapsed and refractory PCNSL. Phase 1 and 2 studies of single-agent ibrutinib have shown an overall response rate of 52–77% in patients with R/R PCNSL, but the duration of response is not satisfactory [31, 32]. In Phase I studies, due to the short duration of response, researchers have combined ibrutinib with other therapies to extend patients’ PFS, including but not limited to rituximab, HD-MTX, and temozolomide [33, 34]. Tirabrutinib, a second-generation BTK inhibitor, showed slightly better results in a Phase 1/2 trial of R/ R PCNSL and is currently approved for the treatment of PCNSL in Japan [35]. Zanubrutinib and Orelabrutinib, two original BTK inhibitors, are also expected to show efficacy in the treatment of PCNSL [36,37,38]. Ibrutinib has a better efficacy in the treatment of relapsed or refractory b cell lymphomas of the central nervous system than peripheral b cell lymphomas, which suggests that the molecular pathogenesis of central and peripheral b cell lymphomas is different. Combined inhibition of BTK and PI3K/mTOR may enhance the therapeutic efficacy of ibrutinib in people with CD79B mutations [39].
Lenalidomide is an immunomodulator with anti-proliferative character and is currently at the heart of several exploratory clinical trials targeting R/R PCNSL. Phase 1 and 2 studies have shown that lenalidomide combined with rituximab has achieved satisfactory results, resulting in an overall response rate of 64-67% and a median progression-free survival of 6-7.8 months [40, 41]. A prospective Phase II study met its primary endpoint and demonstrated clinical, radiological, and biological effects of the lenalidomide and rituximab (R2) regimens in patients with R/R PCNSL and primary vitreoretinal lymphoma [41]. In the more famous FIORELLA trial, researchers use alidomide to treat those elderly patients who have been induced after chemotherapy, maintenance and consolidation, NCCN guidelines also include this drug and ibrutinib into the ranks of rescue treatment drugs, and early clinical trials of various new drugs are also actively carried out [42].
Checkpoint inhibitors and CD-19-targeted CAR T cell therapy are also gradually applied to PCNSL, and related clinical trials are also in full swing [43]. Studies have proved that CAR-T cell therapy is effective in hematological malignancies and some solid tumors, but the efficacy of this therapy for PCNSL is uncertain and is still in the clinical research stage. Professor Miyao et al. reviewed the application of CAR T cell therapy in PCNSL, including the current status of CAR T cell therapy in PCNSL, drug resistance mechanism and future development direction [44]. According to Prof. Miyao, the main disadvantage of CAR-T treatment for PCNSL is the lack of lasting remission, similar to that of systemic lymphoma or leukemia. Novel combination therapies or local CAR-T cell administration in combination with other drugs, including kinase inhibitors or checkpoint inhibitors, are currently under clinical investigation [45, 46]. CAR T cell therapy is expected to change the overall treatment strategy of PCNSL, such as CAR T cell therapy combined with traditional high-dose MTX induction therapy as the first-line treatment of PCNSL, which can improve the response rate and durable response rate of patients, which will reduce the situation of patients receiving high-dose chemotherapy, thereby reducing the risk of associated leucoencephalopathy or neurotoxicity [47]. The exact role of new drugs in PCNSL is currently being investigated, such as potentially effective drug combination applications and their inclusion in first-line treatment protocols, which hold great promise in terms of improving response rates and overall outcomes.
The research of new target drugs opens up broad avenues for the treatment of diseases. Once shown to be safe in combination with each other and with conventional chemotherapy, they hold the promise of substantially improving the response rate to induction therapy, as well as consolidating the duration of response after maintenance therapy, and ultimately improving cure rates, especially for older adults and patients who are not candidates for overly aggressive therapy.In addition, advances in understanding tumor genesis and molecular genetic heterogeneity in PCNSL will improve the targeting of therapies and improve prognosis, and these advances will lay the foundation for the design of clinical trials.
7 Conclusion and outlook
However, despite these impressive advances, supported by the state and the continuous efforts of people in various positions, the disease still poses many challenges for all of humanity. Clinical evidence is accumulating at a rapid rate of high quality due to increased disease detection and patient engagement, but satisfactory clinical studies are still limited. More international and interdisciplinary collaboration is needed in PCNSL research in the future. We summarize the currently available information on the best treatment for PCNSL in the hope of providing a basis for treatment recommendations. In the short run, clinical trials are expected to better determine the role of Cytostatic in the treatment of PCNSL, in addition to HD-MTX, rituximab, ventricle therapy, and HD-ASCT. Since the prognosis of PCNSL is very heterogeneous, the more in-depth the study of this disease and the more thorough understanding of its pathophysiological characteristics will be more conducive to the implementation of individualized treatment, which can significantly extend the survival period of patients, improve the quality of life and reduce the occurrence of adverse reactions. At the same time, we should focus on the research of targeted therapies, so that more efficient and less toxic treatments can be developed.
Availability of data and materials
Not applicable.
Abbreviations
- PCNSL:
-
Primary central nervous system lymphoma
- DLBCL:
-
Diffuse large B-cell lymphoma
- BCL-6:
-
B-cell lymphomas 6
- MUM1:
-
Multiple myeloma oncogene 1
- IRF4:
-
Interferon regulatory factor 4
- BCR:
-
B-cell receptor
- TLR:
-
Toll-like receptor
- Myd88:
-
Myeloiddifferentiationfactor88
- IELSG:
-
International Extranodal Lymphoma Working Group
- KPS:
-
Karnofsky Performance Status
- PFS:
-
Progression-free survival
- HD-MTX:
-
High-dose methotrexate
- WBRT:
-
Whole Brain Radiation Therapy
- BTK:
-
Bruton tyrosine kinase
- PI3K:
-
Phosphatidylinositide 3-kinases
- mTOR:
-
Mammalian target of rapamycin
- IMiDs:
-
Immunomodulatory imines
- NCCN:
-
National Comprehensive Cancer Network
- ASCT:
-
Autologous Stem Cell Transplan
References
Calimeri T, et al. How we treat primary central nervous system lymphoma. ESMO Open. 2021;6(4):100213.
Ostrom QT, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17 Suppl 4(Suppl 4):iv1–62.
Le M, et al. Pretreatment hemoglobin as an independent prognostic factor in primary central nervous system lymphomas. Oncologist. 2019;24(9):e898–904.
Darlix A, et al. Epidemiology for primary brain tumors: a nationwide population-based study. J Neurooncol. 2017;131(3):525–46.
Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410–8.
Swerdlow SH, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90.
You H, Wei L, Kaminska B. Emerging insights into origin and pathobiology of primary central nervous system lymphoma. Cancer Lett. 2021;509:121–9.
Grommes C, et al. Introduction of novel agents in the treatment of primary CNS lymphoma. Neuro Oncol. 2019;21(3):306–13.
Braggio E, et al. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res. 2015;21(17):3986–94.
Chapuy B, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–81.
Zhou J, et al. PIM1 and CD79B mutation status impacts the outcome of primary diffuse large B-cell lymphoma of the CNS. Front Oncol. 2022;12: 824632.
Bataille B, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92(2):261–6.
Eichler AF, Batchelor TT. Primary central nervous system lymphoma: presentation, diagnosis and staging. Neurosurg Focus. 2006;21(5):E15.
Langner-Lemercier S, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18(9):1297–303.
Korfel A, Schlegel U. Diagnosis and treatment of primary CNS lymphoma. Nat Rev Neurol. 2013;9(6):317–27.
Abrey LE, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5.
Ferreri AJ, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266–72.
El Kamar FG, Abrey LE. Management of primary central nervous system lymphoma. J Natl Compr Canc Netw. 2004;2(4):341–9.
Weller M, et al. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro Oncol. 2012;14(12):1481–4.
Ferreri AJ, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217-227.
Ferreri AJM, et al. Long-term efficacy, safety and neurotolerability of MATRix regimen followed by autologous transplant in primary CNS lymphoma: 7-year results of the IELSG32 randomized trial. Leukemia. 2022;36(7):1870–8.
Bromberg JEC, et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2019;20(2):216–28.
Schmitt AM, et al. Rituximab in primary central nervous system lymphoma-a systematic review and meta-analysis. Hematol Oncol. 2019;37(5):548–57.
Nabors LB, et al. Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(11):1537–70.
Houillier C, et al. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the Intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol. 2019;37(10):823–33.
Ostrom QT, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Suppl 5):v1–100.
Hoang-Xuan K, et al. European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro Oncol. 2023;25(1):37–53.
Nayak L, et al. Late relapse in primary central nervous system lymphoma: clonal persistence. Neuro Oncol. 2011;13(5):525–9.
Plotkin SR, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10(17):5643–6.
Rachdi A, et al. Recent advances in the diagnosis and the treatment of primary CNS lymphoma. Rev Neurol (Paris). 2023;179(5):481–9.
Grommes C, et al. Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–29.
Soussain C, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–30.
Lionakis MS, et al. Inhibition of B cell receptor signaling by Ibrutinib in primary CNS Lymphoma. Cancer Cell. 2017;31(6):833-843.e5.
Grommes C, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood. 2019;133(5):436–45.
Okita Y, et al. Histological verification of the treatment effect of tirabrutinib for relapsed/refractory primary central nervous system lymphoma. Exp Hematol Oncol. 2021;10(1):29.
Yuan X, et al. Zanubrutinib plus salvage chemotherapy for relapsed or refractory diffuse large B-cell lymphoma. Front Immunol. 2022;13: 1015081.
Wu JJ, et al. Orelabrutinib-Bruton tyrosine kinase inhibitor-based regimens in the treatment of central nervous system lymphoma: a retrospective study. Invest New Drugs. 2022;40(3):650–9.
Yang C, et al. Orelabrutinib combined with lenalidomide and immunochemotherapy for relapsed/refractory primary central nervous system lymphoma: a retrospective analysis of case series. Front Oncol. 2022;12: 901797.
Nakamura T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42(3):279–90.
Rubenstein JL, et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018;2(13):1595–607.
Ghesquieres H, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective ‘proof of concept’ phase II study of the French Oculo-cerebral lymphoma (LOC) network and the Lymphoma Study Association (LYSA)†. Ann Oncol. 2019;30(4):621–8.
Nayak L, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3.
Frigault MJ, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134(11):860–6.
Miyao K, Yokota H, Sakemura RL. Is CD19-directed chimeric antigen receptor T cell therapy a smart strategy to combat central nervous system lymphoma? Front Oncol. 2022;12:1082235.
Zou R, et al. Long-term complete remission of decitabine-primed tandem CD19/CD22 CAR-T therapy with PD-1 and BTK inhibitors maintenance in a refractory PCNSL patient. Cancer Res Treat. 2023;55(4):1363–8.
Frigault MJ, et al. Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial. Blood. 2022;139(15):2306–15.
Ramadan S, et al. Advances in therapeutic strategies for primary CNS B-cell lymphomas. Expert Rev Hematol. 2022;15(4):295–304.
Acknowledgements
We thank all authors for their contributions to this manuscript.
Funding
The research was funded by the National Natural Science Foundation of China (Foundation number: 8217100680).Key project of Natural Science Foundation of Heilongjiang Province (Fund number: ZD2021H004).
Author information
Authors and Affiliations
Contributions
ZL designed and drafted the manuscript, ZQY reviewed and modified this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the final manuscript.
Competing interests
All authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, L., Zhang, Q. A systematic review of primary central nervous system lymphoma. Holist Integ Oncol 3, 19 (2024). https://doi.org/10.1007/s44178-024-00086-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-024-00086-9