Summary
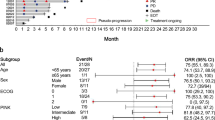
Background. Central nervous system lymphoma (CNSL) is an aggressive lymphoma. Orelabrutinib, an oral Bruton tyrosine kinase inhibitor, is a new treatment strategy for CNSL. This study aims to evaluate the efficacy and safety of orelabrutinib-based regimens in the treatment of patients with CNSL. Methods. Twenty-three patients with CNSL were included in this retrospective study. All patients received the orelabrutinib-based regimen. Efficacy was evaluated based on investigators’ assessment of overall response rate (ORR), complete response/unconfirmed complete response (CR/CRu), partial response (PR), stable disease (SD), progressive disease (PD), duration of response (DOR), progression-free survival (PFS) and overall survival (OS). The safety of orelabrutinib-based regimens has also been evaluated. Results. A total of 17.39% of patients received orelabrutinib-based regimens for consolidation therapy, and 82.61% of patients for induction therapy (4 newly diagnosed CNSL, 15 relapsed/refractory CNSL). In the newly diagnosed CNSL group, the ORR was 100% (1 CR, 1 CRu, 2 PR). The 6-month DOR rate, 6-month PFS rate, and 6-month OS rate were 100%, 100%, and 100%, respectively. Of the 15 relapsed/refractory CNSL patients, five therapy regimens were applied (orelabrutinib, n = 3; orelabrutinib/immunotherapy, n = 3; orelabrutinib/chemotherapy, n = 2; orelabrutinib/immunochemotherapy, n = 6; orelabrutinib/radiotherapy, n = 1). The ORR was 60.00% (4 CR, 5 PR). The 6-month DOR rate, 6-month PFS rate, and 6-month OS rate were 92.30%, 67.70%, and 70.00%, respectively. Twenty-one patients reported adverse events (AEs), and 6 patients experienced grade ≥ 3 AEs. Conclusion. Orelabrutinib-based regimens were efficacious and well-tolerated in patients with CNSL. These combined therapies offer a new potential therapeutic strategy for patients with CNSL.



Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study were included in this published article.
References
Correia CE, Schaff LR, Grommes C (2020) Central nervous system lymphoma: Approach to diagnosis and treatment. Cancer J 26:241–252. https://doi.org/10.1097/ppo.0000000000000449
Narita Y, Nagane M, Mishima K, Terui Y, Arakawa Y, Yonezawa H, Asai K, Fukuhara N, Sugiyama K, Shinojima N et al (2021) Phase I/II study of tirabrutinib, a second-generation Bruton’s tyrosine kinase inhibitor, in relapsed/refractory primary central nervous system lymphoma. Neuro Oncol 23:122–133. https://doi.org/10.1093/neuonc/noaa145
Enblad G, Martinsson G, Baecklund E, Hesselager G, Sundström C, Amini RM, Hagberg H (2017) Population-based experience on primary central nervous system lymphoma 2000–2012: the incidence is increasing. Acta Oncol 56:599–607. https://doi.org/10.1080/0284186x.2016.1270465
Santambrogio E, Nicolosi M, Vassallo F, Castellino A, Novo M, Chiappella A, Vitolo U (2019) Aggressive non-Hodgkin lymphomas: risk factors and treatment of central nervous system recurrence. Expert Rev Hematol 12:787–796
Grommes C, Pastore A, Palaskas N, Tang SS, Campos C, Schartz D, Codega P, Nichol D, Clark O, Hsieh WY et al (2017) Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov 7:1018–1029. https://doi.org/10.1158/2159-8290.cd-17-0613
Lv L, Sun X, Wu Y, Cui Q, Chen Y, Liu Y (2021) Efficacy and safety of ibrutinib in central nervous system lymphoma: a prisma-compliant single-arm meta-analysis. Front Oncol 11:707285. https://doi.org/10.3389/fonc.2021.707285
Chamoun K, Choquet S, Boyle E, Houillier C, Larrieu-Ciron D, Al Jijakli A, Delrieu V, Delwail V, Morschhauser F, Hoang-Xuan K et al (2017) Ibrutinib monotherapy in relapsed/refractory CNS lymphoma: A retrospective case series. Neurology 88:101–102. https://doi.org/10.1212/wnl.0000000000003420
Soussain C, Choquet S, Blonski M, Leclercq D, Houillier C, Rezai K, Bijou F, Houot R, Boyle E, Gressin R et al (2019) Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II “proof-of-concept” iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer 117:121–130. https://doi.org/10.1016/j.ejca.2019.05.024
Lewis KL, Chin CK, Manos K (2021) Ibrutinib for central nervous system lymphoma: the Australasian Lymphoma Alliance/MD Anderson Cancer Center experience. Br J Haematol 192:1049–1053. https://doi.org/10.1111/bjh.16946
Song Y, Song Y, Liu L, Zhang M, Li Z, Ji C, Xu W, Liu T, Xu B, Wang X et al (2019) Safety and efficacy of orelabrutinib monotherapy in chinese patients with relapsed or refractory mantle cell lymphoma: a multicenter, open-label. Phase II Study Blood 134:755. https://doi.org/10.1182/blood-2019-126305
Dhillon S (2021) Orelabrutinib: first approval. Drugs 81:503–507. https://doi.org/10.1007/s40265-021-01482-5
Song Y, Song Y, Liu L, Li Z, Ji C, Xu W, Liu T, Xu B, Wang X (2021) Safety and efficacy of orelabrutinib monotherapy in chinese and western patients with b-cell malignancies. In: The 24th National Congress of Clinical Oncology
Xu W, Song Y, Wang T, Yang S, Liu L, Hu Y, Zhang W, Zhou J, Gao S, Ding K (2021) Updated efficacy and safety results of orelabrutinib in the treatment of relapsed or refractory chronic lymphocytic leukemia/small cell leukemia. Hematol Oncol 39
Song Y, Deng L, Zhang B, Luo H, Zhao R (2021) Preliminary results of orelabrutinib concentrations in peripheral blood and cerebrospinal fluid in patients with relapsed/refractory primary or secondary CNS lymphoma In: The 24th National Congress of Clinical Oncology
Zhu J, Ma J (2021) Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for malignant lymphoma 2021 (English version). Chin J Cancer Res 33:289-301. https://doi.org/10.21147/j.issn.1000-9604.2021.03.01.
Li M, Wang Z-A (2015) New advances in the treatment of relapsed/refractory diffuse large B-cell lymphoma. Chinese J Gen Pract 13:1000–1003
Fridberg J (2011) The American society of hematology education program book. Am Hematol 498–505
Kuitunen H, Kaprio E (2020) Impact of central nervous system (CNS) prophylaxis on the incidence of CNS relapse in patients with high-risk diffuse large B cell/follicular grade 3B lymphoma. 99:1823–1831. https://doi.org/10.1007/s00277-020-04140-0
Health UDO, Services H (2019) Common terminology criteria for adverse events (CTCAE) version 5.0. Published November 27, 2017. In
Han CH, Batchelor TT (2017) Diagnosis and management of primary central nervous system lymphoma. Cancer 123:4314–4324. https://doi.org/10.1002/cncr.30965
Holdhoff M, Mrugala MM, Grommes C, Kaley TJ, Swinnen LJ, Perez-Heydrich C, Nayak L (2020) Challenges in the treatment of newly diagnosed and recurrent primary central nervous system lymphoma. J Natl Compr Canc Netw 18:1571–1578. https://doi.org/10.6004/jnccn.2020.7667
Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE (2006) Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol 24:4570–4574. https://doi.org/10.1200/jco.2006.06.6910
Dhillon S (2021) Orelabrutinib: first approval. Drugs 1–5
Ferreri AJ, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, Torri V, Fox CP, Rosée PL, Schorb E et al (2016) Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 3:e217-227. https://doi.org/10.1016/s2352-3026(16)00036-3
Song Y, Song Y, Liu L, Zhang M, Li Z, Ji C, Xu W, Liu T, Xu B, Wang X (2019) Safety and efficacy of orelabrutinib monotherapy in Chinese patients with relapsed or refractory mantle cell lymphoma: a multicenter, open-label, phase II study. Blood 134:755
Grommes C, Tang SS, Wolfe J, Kaley TJ, Daras M, Pentsova EI, Piotrowski AF, Stone J, Lin A, Nolan CP et al (2019) Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. 133:436–445. https://doi.org/10.1182/blood-2018-09-875732
Wu J, Duan L, Zhang L, Sun Z, Fu X, Li X, Li L, Wang X, Zhang X, Li Z et al (2018) Fotemustine, teniposide and dexamethasone versus high-dose methotrexate plus cytarabine in newly diagnosed primary CNS lymphoma: a randomised phase 2 trial. 140:427–434. https://doi.org/10.1007/s11060-018-2970-x
Markham A, Keam SJ (2019) Camrelizumab: first global approval. Drugs 79:1355–1361
Tam CS, Cull G, Opat S, Gregory GP, Liu A, Johnston AM, Zhao W, Roncolato F, Handunnetti SM, Prince HM (2019) An update on safety and preliminary efficacy of highly specific Bruton tyrosine kinase (BTK) inhibitor zanubrutinib in combination with PD-1 inhibitor tislelizumab in patients with previously treated B-cell lymphoid malignancies. In: American Society of Hematology Washington, DC
Renaud L, Bossard J-B, Terriou L, Cambier N, Chanteau G, Carpentier B, Barbieux S, Wemeau M, Hieulle J, Boyle EM (2020) Treatment with temozolomide and ibrutinib in recurrent/refractory primary (PCNSL) and secondary CNS lymphoma (SCNSL). Blood 136:23–24
Bairey O, Siegal T (2018) The possible role of maintenance treatment for primary central nervous system lymphoma. Blood Rev 32:378–386. https://doi.org/10.1016/j.blre.2018.03.003
Coiffier B, Lepage E, Brière J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235–242
Yu H, Wang X, Li J, Ye Y, Wang D, Fang W, Mi L, Ding N, Wang X, Song Y et al (2021) Addition of BTK inhibitor orelabrutinib to rituximab improved anti-tumor effects in B cell lymphoma. Mol Ther Oncolytics 21:158–170. https://doi.org/10.1016/j.omto.2021.03.015
O’Brien S, Hillmen P, Coutre S, Barr PM, Fraser G, Tedeschi A, Burger JA, Dilhuydy MS, Hess G, Moreno C et al (2018) Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk 18:648-657.e615. https://doi.org/10.1016/j.clml.2018.06.016
Author information
Authors and Affiliations
Contributions
Conception and design, J.W., W.W., M.D., S.M.; Providing materials and samples, J.W., W.W., X.Z., L.Z., S.N., M.D., J.Z.; Data collection, J.W., W.W., L.Z., X.L., L.L., Z. S., X.W.; Data analysis and interpretation, J. W., W. W., X.F., Z. L., Y. C., F. N., J. Y., H.Y., M. Z.; Drafting article, J. W., W. W., X.W., Z.Z.; Administrative support, M. Z.. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the Affiliated Hospital of Zhengzhou University (2021-KY-0886–002).
Consent for publication
Not applicable.
Informed consent
Not applicable.
Research involving human participants and/or animals
Yes.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, JJ., Wang, WH., Dong, M. et al. Orelabrutinib-bruton tyrosine kinase inhibitor-based regimens in the treatment of central nervous system lymphoma: a retrospective study. Invest New Drugs 40, 650–659 (2022). https://doi.org/10.1007/s10637-022-01219-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01219-5