Abstract
In the ecosphere, plants interact with environmental biotic and abiotic partners, where unbalanced interactions can induce unfavourable stress conditions. Abiotic factors (temperature, water, and salt) are primarily required for plants healthy survival, and any change in their availability is reflected as a stress signal. In certain cases, the presence of infectious pathogens such as viruses, bacteria, fungi, protozoa, nematodes, and insects can also create stress conditions in plants, leading to the emergence of disease or deficiency symptoms. While these symptoms are often typical of abiotic or biotic stress, however, there are instances where they can intensify under specific conditions. Here, we primarily summarize the viral interactions with plants during abiotic stress to understand how these associations are linked together during viral pathogenesis. Secondly, focus is given to the beneficial effects of root-associated symbiotic bacteria in fulfilling the basic needs of plants during normal as well as abiotic stress conditions. The modulations of plant functional proteins, and their occurrence/cross-talk, with pathogen (virus) and symbiont (bacteria) molecules are also discussed. Furthermore, we have highlighted the biochemical and systematic adaptations that develop in plants due to bacterial symbiosis to encounter stress hallmarks. Lastly, directions are provided towards exploring potential rhizospheric bacteria to maintain plant-microbes ecosystem and manage abiotic stress in plants to achieve better trait health in the horticulture crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant kingdom is one of the major divisions of an ecosystem where horticultural crops are extremely important to fulfill the continuous food demands of the ancient to the modern world. Changes in climate conditions and environmental factors including extreme temperature fluctuations, water availability, and variations in soil salt concentration are the components that have been considered as major abiotic stress-inducing factors. Usually, abiotic stress conditions even can be capable enough to affect the plant's health/immunity and jointly they prominently induce horrendous effects on plant growth (Gong et al. 2020). The impact of climate change on horticulture can also have ripple effects throughout food management, increasing food prices and reducing food availability for vulnerable populations (Srivastav et al. 2021). Subsequently, climate change can also exacerbate the spread of pests and diseases that can further damage crops and reduce yield. A more or less similar kind of effect must also be expected in the plants during the conditions of deprived or excess water availability. Whereas soil salt and nutrient imbalance could lead to altered plant growth, however, extreme physiological stress conditions can also cause unaccountable biochemical alteration in cellular pathways (Markham and Greenham 2021). Such signatures of abiotic stress appear as deformed and stunted growth patterns in the plants which need attention in terms of employing crop stress management policies to combat abiotic stress conditions in plants (Nepomoceno and Carniatto 2023).
On the other side, infectious plant viruses are also an important part of plants' ecosphere and hold a significant potential to alter crop productivity and yield. It has been noticed during abiotic stress conditions such as elevated temperature, the rate of virus replication and spread can lead to more severe infections and thus cause substantial crop damage (Rubio et al. 2020). Plant virus infections are a major concern in horticulture worldwide, causing significant losses in crop quality as well as yields (Falk and Nouri 2020). Some of the most common plant viruses include Tobacco mosaic virus, Potato virus Y, Cucumber mosaic virus, Plum pox virus, Cauliflower mosaic virus, Tomato yellow leaf curl virus, and Tomato spotted wilt virus (Scholthof et al. 2011). These viruses are spread by insect vector or through mechanical means, and infect the seed material. The impact of plant virus infections on agriculture can be severe and could lead to reductions in crop productivity, loss of market value, and decreased food security. In some cases, plant virus infections can also result in the development of new virus strains/variants, further exacerbating the problem (Rubio et al. 2020). Plant viruses are often transmitted through insect vectors, and noticeably at the time of increased environmental temperatures, which also lead to increased insect populations, resulting in increased disease severity in plants. To combat plant virus infections at elevated temperatures, a variety of strategies can be employed, including the development of virus-resistant and heat-tolerant crops, the use of integrated pest management techniques, and the implementation of quarantine measures to prevent the spread of infected plants (Rubio et al. 2020). Additionally, international organizations and government agencies that are working to monitor should also respond to the plant viral outbreaks to minimize their impact on global food security (Calil and Fontes 2017). The damage caused by plant viruses in horticulture sector can be minimized through proper monitoring, prevention, and control measures. Furthermore, attention to both abiotic and biotic (virus infections) stress must be required simultaneously for better stress management in plants (Ray and Casteel 2022). When plants are exposed to both the stresses together, they interact in various ways. Plants continuously secrete several metabolites that serve a variety of direct and/or indirect benefits; including innate immunity, defence response signaling, plant growth and development, response to environmental stresses, warding off pests and pathogens, promoting plant-microbe symbiosis, and modifying microbial communities associated with hosts biosphere (Erb and Kliebenstein 2020). There are several reports where virus-infected plants become more resilient to abiotic stresses like drought, salinity, etc. In addition, plant microbiomes are also crucial for the development of immunity, disease suppression, nutrition supply, and defence against abiotic and biotic stresses in plants (Haldar and Sengupta 2015). A diversity of secondary metabolites (SMs) has been produced by plants that grow in different areas and/or fluctuating growth conditions (Mishra et al. 2021). An interesting question is whether the production of such SMs could be able to dictate the diversity of plant rhizosphere-associated bacterial symbiotic consortia. If yes, then bacterial association and cross-talks between the plant and bacterial molecules have the potential to encounter abiotic stress outcomes in the plants and could be able to improve crop health in all respects (immunity, yield, and nutrition values). Conceptually, abiotic stress, virus pathogenesis (biotic stress), and microbial symbiosis are not easy to relate together due to the coherence and interference of innumerable factors that are undergoing continuously in plants during such multipartite associations and stress conditions. Therefore, for a comprehensive understanding, it is essential to delve into the intricate cellular and molecular interactions that take place between plants, pathogens, and symbionts. Such information could be useful to understand multipartite associations of plants during normal as well as stress conditions. Therefore, with the excitement to know more about the curious and furious fact of abiotic stress with overlaps of infectious virus-induced biotic stress, their respective and/or cumulative effects on the plants are discussed as the first major section of the article. Whereas normal and concurrent interactions during abiotic stress of plant with root symbiotic commensal bacteria are thoroughly discussed in the second section of the article in light of recent and relevant literature. An overview of viral pathogen-plants-symbiotic root microbes has also been shown in Fig. 1.
Abiotic stress and virus infections in plants
Abiotic stress exerts an unconstructive impact of lifeless factors on livelihood. These include temperature, drought, salinity, and other extremities that play a pivotal role in horticulture as they affect various stages of crop growth and development (He et al. 2018). Ideal temperatures for plants vary depending on the species, but in general, warm temperatures promote germination, growth, and flowering, while extreme temperatures can lead to stunted growth, reduced yields, and even death (Bita and Gerats 2013). Additionally, temperature affects soil and water conditions, which in turn affects plant growth and productivity (Seleiman et al. 2021). Hyper-salinity and drought are among the primary causes of crop loss worldwide. In general, a suitable range of temperature, salt, and water is required to sustain optimal growth and yield of crops (Ma et al. 2020). All these abiotic stresses influence plant growth in natural and diseased conditions. However, certain common host factors play an important role during stress induced by high temperatures, salinity, and drought with biotic stress like viral infections (Audil et al. 2019).
Viruses are intracellular, obligate pathogens that affect almost every biological entity present on the planet (Guo et al. 2019). Plant virus infections are a major concern in agriculture worldwide, causing remarkable losses in the quality and yields of crops. Some of the most common plant virus infections include that caused by Potato virus X, Tobacco mosaic virus, Potato virus Y, Cucumber mosaic virus, Tomato yellow leaf curl virus, Cauliflower mosaic virus, Plum pox virus, Brome mosaic virus, and Tomato spotted wilt virus (Scholthof et al. 2011).
Abiotic factors like temperature, salinity, and drought play pivotal roles in shaping the virus's evolution, virus spread, eradication, etc. (van Munster 2020). On the contrary, plant viruses can have a significant impact on crop productivity and yields, particularly during different abiotic stresses. Warmer temperatures can increase the rate of virus replication and spread, leading to more severe infections and greater crop damage (Velásquez et al. 2018). However, in some cases, higher temperatures sustain the viral infection but over time lead to decreased accumulation of viral load (Roberts et al. 2018). Drought usually stimulates plant virus infections, further exacerbating the problem (Seleiman et al. 2021). Salinity stress was also demonstrated to favour the propagation of viral pathogens within the plants (Prasad et al. 2022). In the subsequent sections, we have discussed the role of various abiotic stresses on plant viral diseases. An overview of the defensive systems, and regulatory network affected by multi-stress (virus infections and abiotic) conditions is presented here with practical potential that could deliver resilience, resistance, and subsequently higher yield.
Many abiotic stresses affect the overall plant growth like temperature, drought, heavy metals, ultraviolet radiations, and salinity (He et al. 2018). Among these, three stress factors i.e., temperature, drought, and salinity hugely affect plants that either prone them to viral infections or make plants resilient towards them. A few such important stress factors affecting plant mechanisms are listed in Table 1.
Plant-virus interactions during temperature stress
Plants compete strongly in an environment those have a sympathetic environmental temperature. Plant growth and development are unnatural with fluctuations in optimum temperature that directly affect their cellular morphology and cellular signalling (Hatfield and Prueger 2015). Elevated temperature impacts the plant morphology and shows phenotypic effects from sweltering of stem and leaves, shortening of life process, ablation of plant parts, inhibited growth of roots and shoots, sexual cells becoming sterile, and also affecting fruit quality. During higher temperatures, the physiology of cells also gets affected and is mainly characterized by a reduction in photosynthetic and respiratory rate, membrane permeability and fluidity changes, etc. (Desaint et al. 2021).
Temperature plays an important role in overall plant development and higher temperatures are known to act as pro-viral factors in the early stages of infection with virus eradication in later stages. Infection of Capsicum chlorosis virus promoted viral replication at the commencement of infection and recovered systemically at the subsequent stages because of vigorous incitement of RNA silencing machinery in the host (Tsai et al. 2022). High viral accumulation of Peanut stunt virus was recorded at higher temperatures (27 °C) that decreased tremendously later, contrary to high viral accumulation both at initial and later stages of infection at 21°C (Obrępalska-Stęplowska et al. 2015). Replication of Barley yellow dwarf virus (BYDV) was boosted at a higher temperature (21 °C), which also caused the emergence of early virus symptoms and a high viral titer in Triticum aestivum cv. Yitpi (Nancarrow et al. 2014). In general, there are two possible outcomes in different host-pathogen combinations about heat stress, 1) either the virus accumulates largely, or 2) the host recovers from infection. Some common regulatory mechanisms were highlighted when plants were subjected to both heat and viral infections. Proteome analysis highlighted a significant decrease in the accumulation of proteins participating in photosynthesis (like rubisco, ferredoxin-NADP reductase) and carbohydrate metabolism (like Fructose-1,6bisphosphatase) at different temperatures (Obrępalska-Stęplowska et al. 2015). However, heat-stressed plant species have similar proteins affected (Zhao et al. 2018). It has been reported that there are elevated levels of heat shock protein (HSPs) dynamics in potato plants when they were subjugated to either warmth or to Potato virus Y (PVY) infection (Table 1). Investigations yet again decorated that PVY infection and torrid heat stress have assured the existence of common regulatory mechanisms (Makarova et al. 2018). These findings have important implications because plants respond by changing protein expressions to stresses such as heat and viral infections which is a plant's adaptation behaviour. Subsequently, the accumulation of these proteins affects the virus titer and hence infection.
Some reports have mentioned that host resistance is altered under increased temperature (Desaint et al. 2021). Resistance is heritable immunity that minimizes damage induced by plant pathogens. During the interaction of Nicotiana xanthi and Tobacco mosaic virus (TMV), the hypersensitive response (HR)-type resistance was overcome when plants were kept at higher temperatures. This was evidenced by the downregulation of superoxide and other antioxidant enzyme activity that were associated with the suppression of HR-type resistance (Király et al. 2008). It was also reported that infection of TMV in N. tabacum increased with increasing temperature to 28◦C. In another report, Tomato spotted wilt virus (TSWV) and Potato virus X (PVX) co-infection, the procedure of HR abolishment was similar and quite noticeable (Wang et al. 2009; Chung et al. 2018). It was implicated that temperature fluctuations either change R-gene protein conformation or the intrusive virus destabilized R-genes at higher temperatures promoting virus multiplication. It has been reported that elevated temperatures lead to the denaturation of existing proteins or the misfolding of newly synthesized proteins (Volkening et al. 2019). Solanum lycopersicum HSPs interact with TYLCV proteins and promote virus accumulation. HSPs are expressed in response to heat so that mature proteins are folded appropriately to perform necessary functions. The segregation of HSPs between virus and heat strain retaliation led to incompetent implementation of the protein due to that heat stress response efficiency was decreased as detected in TYLCV-infected tomatoes prone to high temperature (Prasad et al. 2019a). High temperatures and dryness dramatically boosted Arabidopsis' sensitivity to TuMV by inhibiting PR and R genes (Prasch and Sonnewald 2013). It has been demonstrated that the NBLRR (N) protein, which detects the TMV signal, underwent a conformational shift after TMV infection. As a result, plant cell death (PCD) that limits viral replication and propagation was not able to start the signal transduction chain. The infection altered the N gene's role in resistance because less N protein accumulated in the nucleus, which inhibited downstream signalling connected to hypersensitive response suppression. In the course of TMV infection of tobacco and Tomato Spotted Wilt Virus (TSWV) infection of tomato, HR response and R-gene mediated plant defence responses dependent on heat has become reduced (Zhu et al. 2010). High temperature (32°C) has also been shown to disrupt capsicum and tabacum plant resistance to TSWV mediated by heterozygous Tsw gene locus, causing systemic dissemination and the emergence of necrotic signs. Hence, in some virus-host combinations, changes in hosts are induced by viruses for their survival (Table 1) (Llamas-Llamas et al. 1998; Moury et al. 1998).
Viruses are obligate parasites and are dependent on host factors for their survival. Their infection leads to the activation of a defence pathway, known as RNA silencing, at the viral entry (Prasad et al. 2019a). The double-stranded RNA replication intermediates are targeted by a class of endonucleases known as DICERs that target them and reduce them to 21-24nt sized small interfering RNAs. These siRNAs bind to their cognate viral RNAs and silence them. RNA silencing response is suppressed under high temperatures and increases plant susceptibility to pathogen infections (Travella et al. 2006). Many studies indicated depletion in viral titer in the new leaves due to the accumulation of siRNAs (Sahu et al. 2010).
The generation of these virus-induced siRNAs is affected by temperatures that consequently enhance/reduce the viral load. N. benthamiana plants infected by Tomato ringspot virus (ToRSV) displayed symptoms earlier when kept at 27 °C compared to 21°C, due to the effective generation of vsiRNAs. However, plants recovered from infection 8 days post inoculation (dpi) at 27°C, with symptomless leaves containing fewer viral proteins (Ghoshal and Sanfaçon 2014). In viral infections of the fruit trees, it has been shown that low temperatures favour the viral infection as the host's silencing mechanism is arrested at lower temperatures (Tatineni et al. 2009). Brassica campestris plants infected with TuMV when kept at a high temperature accumulate more viral coat protein. Up to 23°C, rising temperature causes a proportional increase in the rate of systemic infection (Chung et al. 2015). Contrarily, Cotton leaf crumple virus (CLCrV) infection in Gossypium hirsutum plants displayed enhanced virus silencing when plants were kept at higher temperatures (30°C/26°C day/night), even though endogenous silencing of the gene is diminished at 30 °C and 26°C (Table1) (Tuttle et al. 2008).
With the recent advancements in biotechnology, plant virus infections are now reported to endow plants with properties that allow them to combat different stresses, though in general, it has been discovered that pathogenic infections reduce a plant's ability to withstand stress due to abiotic factors (Márquez et al. 2007). For example, tomato plants with ToYLCV infection were sensitive to high temperatures to a greater extent. Reduced responsiveness of HSFs and HSPs has been linked to this enhanced heat vulnerability (Anfoka et al. 2016). In another report for illustration of this mutualistic interaction, the Pigeon pea sterility mosaic virus (PPSMV)-I and -II-infected pigeon pea plants have high-temperature stress tolerance (Kumar et al. 2017). It was claimed that B. vulgaris with CuMV infection had improved cold tolerance (Xu et al. 2008). Earlier it was believed that viruses were just parasites that harmed plants and reproduced by utilizing the resources and chemicals of their hosts. The positive functions of some viruses for their plant hosts, however, have been detailed by fresh information in recent years. According to one definition, viruses interact mutually with plant cellular systems, providing some abiotic stress protection for plants (González et al. 2020). One of the early research in this field demonstrated how RNA viruses like the Cucumber mosaic virus (CMV) could make plants resistant to cold and drought (Xu et al. 2008). Abiotic stress is also exerted at high temperatures which can further result in an elevation in the occurrence of insect vectors that carry with them a load of pathogens. With the increasing global temperatures, increasing the yield by the development of plants that can withstand infections and higher temperatures is necessary to meet increasing food demand.
Plant virus interaction during drought stress
Drought is a serious abiotic stress that affects plant growth and proportionally crop output (Franklin et al. 2016). It is one of the primary plant stressors, affecting fitness or sometimes even leading to mortality depending on its intensity and duration (Xu et al. 2008). The stress induced by water not only interferes with plant-virus interactions but also affects plant-insect interactions, which further exacerbates virus spread (Davis et al. 2015; Blanc and Michalakis 2016). Drought and pathogen stress have been found to be detrimental to plant growth and productivity (Sinha et al. 2016). Pathogen infection has also been demonstrated to influence plant responses to water scarcity. Drought can have a good impact and reduce illness levels, but it also increases disease susceptibility in many circumstances (Prasch and Sonnewald 2013; Ramegowda et al. 2013; Kissoudis et al. 2014). The virus-infected plants may have reduced basal defences that could make them more susceptible to drought stress (Hulten et al. 2006). For instance, when Sweet corn plants infected with Maize dwarf mosaic virus (MDMV) were concurrently exposed to drought stress, they demonstrated a greater drop in leaf area, ear weight, and height of plant than non-infected plants (Wang et al. 2012).
Several studies also reported beneficial effects of virus infection that make plants resilient to drought stress. In one such case, Arabidopsis plants infected with TuMV, were passed through serial passages that induced virus evolution, under normal watering conditions and separately under drought stress. The results demonstrated that the drought-evolved TuMV increased Arabidopsis efficiency to withstand drought tolerance. The findings demonstrated how a virus adapted to stress conditions and subsequently benefitted the host plant. Recent findings also highlighted the induction of more drought tolerance in plants with serious virulent infections and modest tolerance with superficial viral infection, allowing the plant to complete its life cycle and produce viable offspring. In addition, virus infections minimize water loss through reduced transpiration due to partial stomatal closures (Xu et al. 2010). It was anticipated that higher viral-induced tolerance is invariably followed by improved biological fitness (Aguilar et al. 2017). Interestingly, TYLCV infection in tomato plants where virus-infected plants were more resilient when compared to uninfected plants (Mishra et al. 2022). Virus-infected plants under drought stress have boosted resistance mechanisms compared to non-infected plants. Co-infection of Potato virus X (PVX) and Plum pox virus (PPV) in N. benthamiana and Arabidopsis displayed improved drought tolerance by boosting salicylic acid levels further improving photosynthetic performance and enhanced activity of antioxidant enzymes (Khalvandi et al. 2021). N. benthamiana plants infected with Brome mosaic virus (BMV), Cucumber mosaic virus (CMV), and TuMV revealed belated emergence of leaf drooping and stem dryness compared to non-infected drought-stressed plants (Gentleman et al. 2004). The infected plants accumulated more osmoprotectants such as glucose, fructose, and sucrose (Xu et al. 2010). The enhanced amounts of these metabolites in virus-infected plants primed plants to cope with abiotic stress. The metabolic and physiological changes caused by viral infection may have mitigated the impacts of drought stress and hence conferred combined stress tolerance.
Plant virus interaction during salt stress
Abiotic stressors have a significant impact on plant growth and yield (Gharsallah et al. 2016). High salt concentrations in saline soil prevent plants from absorbing water and nutrients (Gong 2021). Due to this imbalanced osmotic stress and ionic stress are induced (Zhu 2002). Collectively, physiological and molecular changes brought on by salt stress prevent plants from growing by lowering the amount of resources available, limiting photosynthesis, and suppressing cell division and growth (van Zelm et al. 2020). Excess sodium-induced changes in calcium levels and reactive oxygen species (ROS) are early indicators that trigger a salt stress response (Park et al. 2016). Plant encountering salt stress enhances the cytosolic calcium level due to ion and osmotic stress brought on by salt stress. Calcium activates Ca2+ sensors and further serves as a crucial secondary messenger (Yuan et al. 2014; Zhang et al. 2020).
Plant viral infections have different outcomes in virus-host interactions under salt stress. According to some recent findings, the host plant's viral load rises as a result of the plant's cells sensing and response to abiotic stress. As a result, increased field crop losses could result from the interaction of abiotic stress and viral infection (Zhao et al. 2021). In viral infections, calcium has been shown to play a role in virion generation, maturity, and stability, as well as viral entrance and replication. Whether through pumps and/or channels on the plasma membrane, viral proteins regulate calcium flux and also cause calcium release from internal reserves (Zhou et al. 2009). Several viral proteins trigger calcium-responsive transcription by interacting with cellular calcium receptors. In potatoes infected with Potato virus Y (PVY), the transcript levels of genes related to photosynthesis, perception, signalling, and defensive responses were changed (Baebler et al. 2009). At the onset of infection in susceptible potatoes, genes involved in calcium-signalling were up-regulated, suggesting that calcium may be crucial for the development of potyvirus infection. In another report on Pepper yellow mosaic virus (PepYMV) infections, it was found that 4.9% of the genes up-regulated were related to calcium-mediated signal transduction (Alfenas-Zerbini et al. 2009). Salinity stress has been shown to encourage the expansion of bacterial and viral diseases inside plants, and increased rate of infection (Kissoudis et al. 2016; Varela et al. 2019).
Recent findings reported the resistant-breaking mechanisms in plants under salt stress. In tomato TYLCV combination, regardless of whether the tomato genotypes were virus susceptible or not, salinity stress caused a considerable viral accumulation. The sensitivity of the tomato-susceptible genotype to TYLCSV was significantly increased, and its defence appeared to be weaker. The efficacy of the Ty-1, Ty-3, and Ty-5 loci-mediated TYLCSV resistance was significantly reduced by salt stress. Factors related to salinity decreased the efficiency of the tomato TYLCSV resistance. An impervious genotype of cowpea when treated with salt stress and then infected with Cowpea severe mosaic virus (CpSMV) has exhibited critical symptoms. It was hypothesized that salinity-related disturbances in the redox metabolism contributed to the virus's more severe symptoms (Varela et al. 2019). Furthermore, it was discovered that N. benthamiana plants were more vulnerable to Potato virus A (PVA) infection when exposed to salinity stress (Suntio and Mäkinen 2012). It was also been reported that during the conditions of salt stress, seedlings of N. benthamiana obtained from mock-inoculated plants depict elevated inhibition of root growth as compared to those that were infected with PVX (Potato Virus X). Furthermore, corresponding fresh weights of progenies derived from PVX were enhanced by 1.6 fold when compared with those which were mock inoculated in the presence of NaCl and Mannitol respectively (Hernández-Walias et al. 2022).
One of the main approaches utilized by viruses to create favourable interactions with plant hosts is a reduced rate of photosynthesis, which further reduces the accumulation of ATP and NADPH required for plant defence (Bolton 2009). Such defence-compromising mechanisms induced by salt stress, result in the loss of viral resistance in plants. More severe symptoms of the virus have been proposed to be caused by salinity-related changes in redox metabolism (de la Torre et al. 2014).
Contrary to the increased susceptibility of plants to viral infections under salt stress, resistance/tolerance has also been imparted in a few cases. Systemic Eggplant mottled crinkle virus (ECMV) accumulation was dramatically reduced in N. benthamiana plants when they were exposed to salt stress before infection. In another such example, the overexpression of an insect Flock house virus B2 protein increased the ability of rice and tobacco to resist salt stress by enhancing stomatal performance and photosynthesis (Table 1) (Sinha et al. 2021). The connection between biotic and abiotic stress factors and plant responses possibly hypothesized the existence of common defence routes for plant adaptation to challenging situations (Moldakimova et al. 2012) (Fig.2).
Plant-symbiotic bacteria interactions during abiotic stress
Plant-symbiotic bacteria interactions during temperature stresses
High temperatures are a type of usual stress in plants where conditionally several root associate soil microbes conduct a molecular cross-talk to provide dictations for survival adaptations. Every plant-pathogen interaction has a disease-causing temperature range such as Globodera pallida nematodes infect the potato plants at 15 °C (Jones et al. 2017) whereas Xanthomona soryzae colonize in the plants at 35 °C as well as 27 °C (HORINO et al. 1982). However, such kind of temperature variations and the infectivity potential of bacteria (beneficial and/or harmful) are difficult to predict. It has been noticed that effectors triggered immunity (ETI) activation at higher temperatures reduce plant defence hormone salicylic acid (SA) and SA-associated defence gene expression, making Arabidopsis more susceptible to pathogens such as Pseudomonas syringaepv than Tomato (Li et al. 2020). Temporary heat stress decreases the pattern-triggered immunity (PTI) signalling and Pst DC3000 resistance in Arabidopsis (Cheng et al. 2013; Janda et al. 2019). PTI-responsive genes, MAPK, and BIK1 phosphorylation were also be reported to activated more strongly even after a brief exposure to increased temperature (Cheng et al. 2013). Heat stress can influence unstressed progeny's immunity through epigenetics (Liu et al. 2019). High temperatures suppress type IV secretion-associated pilus formation and virulence gene expression in Agrobacterium infections (Jin et al. 1993; Baron et al. 2001). At higher temperatures, Pectobacterium atrosepticum produced more plant cell wall-degrading enzymes, quorum-sensing signals, and disease development (Hasegawa et al. 2005). Microorganisms with altered respiration grow faster at high temperatures which could increase microbial soil respiration (Karhu et al. 2014). Soil microbes use organic matter in a temperature-dependent manner (Frey et al. 2013). Soil warming and drought indirectly affect rhizosphere nutrient uptake and carbohydrate exchange (Newsham et al. 1995). Rhizosphere bacteria and endophytes reduce plant temperature stress and promote crop growth in different climates, soils, and temperatures. However, soil and temperature status may affect plant-beneficial bacteria interactions (Bilal et al. 1993; Javed and Arshad 1997; Bashan 1998). Mycobacterium sp. 44, P. fluorescens, and Pantoea agglomerans strains isolated from a semi-continental climate significantly increased winter wheat root and shoot growth at 16°C compared to 26°C. M. phlei strain MbP18 and Mycoplana bullata MpB46, both from semi-arid climates, performed well under both conditions, indicating genotype-specific environmental preferences (either loamy or sand). It was demonstrated that desert woody legume Prosopisgla ndulosa nodule Rhizobia grows better at 36°C than the 26°C (Waldon et al. 1989). Bacteria colonizing different sites may also react differently to environmental conditions. Burkholderia phytofirmans PsJN showed a temperature increase from 10 to 30°C. Reduced tomato rhizosphere colonization of this strain, but endophytic abundance remained unaffected (Pillay and Nowak 1997). Rhizosphere and endophytic bacteria can induce a systemic response to reduce plant stress from temperature or drought (Lichtfouse 2009; Yang et al. 2009). Heterotrophs' carbon incorporation has been noticed to be increased even at a little rise (40 °C) for a short time, which possibly could be due to phytoplankton's instant release of photosynthate. Attachment and higher temperatures accelerate heterotrophic incorporation of phytoplankton-derived dissolved organic carbon (DOC). Warmer temperatures double the DOC incorporation by free-living heterotrophs and quadrupled it by attached heterotrophs, showing the importance of attachment in transferring recently fixed carbon to secondary consumers. Hence, one can easily understand that any temperature changes going to affect the mutual as well as opportunistic bacterial associations with plants which will again also depend on the plant species. So, such kind of knowledge going to be the gold standard at the time of selecting the bacterial species to get the maximum benefit in the type of crop as well as high-temperature stress management.
Plant-symbiotic bacteria interactions during salt stresses
A horrible situation encountered by the plants during the salt stress. Sessile plants experience many environmental stresses throughout their development. Salinity threatens 25%-33% of global crop production (Kumar et al. 2022). Salinity stress affects 85% of the planet, according to the FAO (Food and Agriculture Organization of the United Nation, 2022). Bad agricultural practices (high salt content water used for irrigation and fertilization) and saline water from the sea, rivers, etc., especially in arid and semiarid regions, cause soil salinity (Zhang et al. 2021). Climate change, drought, and rising sea levels often make soil saline (Kumar et al. 2022). High osmotic stress impairs plant nutrient uptake and transport (Farooq et al. 2015). Salinity stress negatively impacts plant physiological development (Mahawar and Shekhawat 2019) and soil microbiota also compromises the soil health (Dubey et al. 2022). yet, plants can capable of competing salinity stress by altering a variety of morphological, physiological, and molecular responses (Zhao et al. 2020), such as increasing phytohormone and osmoprotectant synthesis, antioxidant activity, Na+ homeostasis, and compartmentalization (Arif et al. 2020). Cohabiting with diverse halotolerant (HT) microorganisms improves plant growth, stress tolerance, and nutrient uptake, restoring crop yield (Etesami and Beattie 2018; Etesami and Glick 2020). Rhizosphere, epiphytes, and endophytes are the types of plant-interacting HT microorganisms (Andrews and Harris 2000; Hardoim et al. 2015). Accountable reports showed that plant species can colonize their self-bacterial populations (Kuske et al. 2002). Plants used three main strategies to survive in saline environments: osmotic Na+/Cl- stress, exclusion, and accumulation. HT-PGPR, diverse saline soil microbes, improves plant adaptation to salinity stress. HT-PGPR increases the saline-agro-ecosystem productivity directly by producing exopolysaccharides (EPS), siderophores, volatile organic compounds (VOCs), compatible osmolytes, and phytohormones (Bhat et al. 2020) or indirectly by regulating stress-related gene expression and phytopathogen effects (Prasad et al. 2019b). Rhizobium, Arthrobacter, Flavobacterium, Alcaligenes, Pseudomonas, and Azospirillumare capable of improving the crop salinity tolerance (Saghafi et al. 2019; Kumar Arora et al. 2020), whereas, stressed HT bacteria form biofilms as well as produce EPSs (Haque et al. 2022).
The bacterial growth phase, nutrient medium composition, stress stimuli, pH, and temperature affect HT-PGPR EPS production (Kumar Arora et al. 2020). EPS makes up 40-90% of bacterial weight in saltwater (Sunita et al. 2020). The rhizosheath, which also acts as a carbon source, improves the nutrient and water uptake from the rhizosphere and protects the plant from ionic salts and phytopathogens (Mishra and Arora 2018; Kumar Arora et al. 2020). It was examined how EPS-producing halotolerant Enteroba ctercloacae and B. drentensis increased crop plant water uptake and nutrient availability to improve salt-stressed mung bean growth (Mahmood et al. 2016). EPS promotes soil aggregation, humification, water retention, nodulation, quorum sensing, and microbial diversity that protects plant cells from desiccation in saline environments (Kumar Arora et al. 2020). Antioxidants in EPS can protect against salinity-induced oxidative damage (Sunita et al. 2020). Volatile organic compounds (VOCs) are produced by HT-PGPR under stress (Sunita et al. 2020). VOCs stimulate siderophores, osmoprotectants, phytohormones, HKT1/K+ transporters, virulence factors, and bacterial motility in the plant-microbe interaction to help stressed plants grow and adapt (Bhat et al. 2020). Salinity-stressed crops also face low availability of soluble ferrous ions (Fe2+), which is mostly determined by soil pH. Alkaline pH (pH >6.5) in saline soils oxidises Fe2+ to ferric (Fe3+) and reduces plant iron availability. Salt-stressed crops' Fe needs are met by Fe3+ HT-PGPR's and siderophores. HT PGPR can help plants accumulate compatible osmolytes (amino acids, soluble sugars, and polyols) in a saline environment to reduce osmotic stress, and maintain high turgor pressure and cytoplasmic ion equilibrium. To reduce water stress in plants HT-PGPR upregulates the osmolyte biosynthesis genes (mainly proline), and controls stomatal conductance and transpiration. Bioinoculation of capsicum with a halo tolerant PGPR (B.fortis SSB21) increases the proline synthesis and stress-related gene expression including the pepper pathogen-induced protein gene (CAPIP2), putative ketoacyl-ACP reductase (CaKR1), pepper osmotic-like protein 1 (CaOSM1), and pepper class II basic chitinase (CAChi2). Salt-tolerant PGPR upregulates the phytohormone synthesis genes, mainly IAA, to compensate for plant growth hormones, change root morphology, and exclude excess ionic salts. In vitro studies showed that HT-PGPR increases IAA production in plants, which reduces tap root growth, elongates root hairs, and increases lateral root number and length. Thus, water and nutrient availability and uptake improve crop growth. In Coleus forskohlii, P. putida and Maltophilia increased IAA, gibberellic acid, and cytokinin production. Under NaCl stress, Pseudomonas sp. Increases the gibberellin and cytokinin production in Glycine max and Zea mays plants respectively. PGPR synthesizes and regulates stress hormones ABA and ethylene in addition to other growth hormones. HT strain Dietzia natronolimnaea STR1upregulates the expression of ABA signaling cascade genes like ABA response elements (TaABARE) and 12-oxophytodienoate reductase 1 (TaOPR1), which stimulates the salt stress-induced gene TaST in T. aestivum (Bharti et al. 2016). Ethylene, another stress hormone, increases salinity tolerance but limits plant growth and productivity. HT-PGPR secrete ACC deaminase, which metabolizes ACC (ethylene precursor) into alpha-ketoglutarate and ammonia, inhibiting plant ethylene synthesis (Bhat et al. 2020). Certainly, it has been indicated that the inoculation with plant growth promoting Rhizobacteria containing ACC-deaminase could be a useful approach for improving growth and yield of maize under salt-stressed conditions (Nadeem et al. 2009). In addition to producing plant-beneficial metabolites HT-PGPRs also regulate the Na+/K+ homeostasis and modulate the expression of salt-tolerant genes like salt overly sensitive (SOS), high-affinity K+ transporters (HKT), Na+/H+ antiporter (NHX), aquaporins (AQPs), and antioxidants to protect plants from salinity stress. HT-PGPR B. subtilis (GB03) reduces Na+ uptake in the halophyte grass Puccinellia tenuiflora by upregulating PtHKT1;5 and PtSOS1 and downregulate the PtHKT21 (Wang et al. 2015). In saline plant (T. aestivum) the bacteria B. subtilis GB03 execute similar mechanism (Zhang et al. 2014). In salt-stressed Zea mays, HT-PGPR B. megaterium upregulates the aquaporin genes (ZmPIP1-1 and PIP2), increasing water uptake (Marulanda et al. 2010) by modulating antioxidant expression and activity, HT-PGPR increases host plant salt tolerance (Kumar Arora et al. 2020). The HT-PGPR boosts crop growth and yields in normal and stress conditions better than synthetic agrochemicals. Many PGPR-based bioformulations and products are in development or present in the market for applications. Laboratory screening assays and field trials are essential to PGPR production (Backer et al. 2018). Commercial PGPR inoculants did not promote crop growth in agricultural fields like those under controlled laboratory conditions. Climate change hinders field PGPR performance. Climate change affects plant physiology and plant-associated microbial community diversity, abundance, colonization, and activity independently as well as jointly (Tabassum et al. 2017). It is quite obvious that no potent commercial inoculant works in all ecological zones, therefore, climate also affects the PGPR efficiency (Liu et al. 2022). Bioinoculants must also consider crop variety and PGPR strain. PGPR strains or consortia affect crop growth and yield differently depending on the cultivar. PGPR performance depends on carrier choice. Inappropriate carriers reduce rhizosphere bacteria survival and efficiency. Another factor is microbe consortia compatibility. Many bacterial strains interact antagonistically, reducing field PGPR efficiency (Tabassum et al. 2017). Halophytes store plant-growth-promoting halotolerant bacteria. High phosphorus-solubilizing halotolerant PGPR bacteria (sp.YCWA18) isolated from the place Daqiao Kushneria saltern sediment on the eastern coast of China capable of growing on a solid medium with 20% (w/v) NaCl (Zhu et al. 2011). PGPRs that were found to be halotolerant based on their ability to tolerate 2-25% NaCl, including B. pumilus, P. mendocin, Arthrobacter, Halomonas, Nitrinicola lacisaponensis, and with other PGP traits like phosphorus (P) solubilization and the ability to produce IAA, siderophores, and ACC deaminase have also been isolated (Tiwari et al. 2011). Due to poor solubility and soil fixation, only 0.1% of P is available to plants (Goldstein 1986). Inorganic NPK fertilizers and saline irrigation increase soil salinity. Phosphate-solubilizing HT PGPRs increase plant P availability without increasing soil salinity. PGPRs can solubilize insoluble phosphates through chelation, ion exchange, and acidification by secreting low-molecular-weight organic acids (Sharma et al. 2013; Etesami 2018). Under field salinity stress, wheat inoculated with B. aquimaris plant increases P content (Upadhyay and Singh 2015).
Plant-symbiotic bacteria interactions during drought stresses
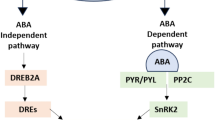
Drought conditions in plants directly as well as indirectly affect the root bacterial communities by modulating moisture availability, soil chemistry, and plant phenotypes. Due to resource limitation, total bacterial biomass decreases under drought (Hueso et al. 2012; Alster et al. 2013) and in more arid soils along a precipitation gradient (Bachar et al. 2010). In moisture-limited soils, the presence of bacteria Proteobacteria, Verrucomicrobia, and Bacteroidetes decreased and the genera of Firmicutes and Actinobacteria found to be increased (Barnard et al. 2013; Bouskill et al. 2013; Acosta-Martínez et al. 2014; Curiel Yuste et al. 2014). The soil community composition shifts have several possible causes. First, Gram-positive and Gram-negative bacteria may have different drought susceptibilities due to substrate preference and metabolic capacities. Drought stress conditions can be oligotrophic-nutrient-poor but could be oxygen-rich. Oligotrophs (slow-growing microbes) usually grow in poor conditions. In droughted soils, oligotrophic bacteria proliferate because of complex plant polysaccharide degradation genes that are more abundant than those targeting oligosaccharides (Bouskill et al. 2016b; Martiny et al. 2017). Gram-positive bacteria are metabolically "hardy" and can use inorganic nitrogen to produce extracellular enzymes that degrade complex organic compounds, which are abundant in droughted soils (Treseder et al. 2011). Actinobacteria genera can use recalcitrant carbon sources and are abundant in arid, nutrient-poor soils (Connon et al. 2007; Curiel Yuste et al. 2014; Mohammadipanah and Wink 2016; Hartmann et al. 2017). Gram-negative bacteria are copiotrophs, preferring labile carbon compounds and organic nitrogen, especially in the form of plant root exudates (Balasooriya et al. 2014). Under well-watered conditions, Gram-negative bacteria incorporated almost ten times as much plant-derived carbon as Gram-positive bacteria (Fuchslueger et al. 2014). Plants mechanistically close the protein channels to prevent sugar transport to the rhizosphere during drought osmotic adjustment. Bacterial activity levels may be a third cause of drought-induced community composition changes. The "Birch effect" occurs after rewetting dry soils, where bacterial activity and CO2 efflux increase dramatically (Blazewicz et al. 2014; Armstrong et al. 2016). Wetter soils have less gaseous diffusion, creating a more anaerobic environment (Liptzin et al. 2011) and higher bacterial gene abundances for genes involved in anaerobic fermentation (Schwartz et al. 2007), O2 limitation, and denitrification (Bouskill et al. 2016b). Under drought, soil bacterial activity decreases as microbes die or go dormant (Jensen et al. 2003; Alster et al. 2013), but complex carbon degradation gene abundance increases (Bouskill et al. 2016b). In addition, drought can trigger presented microbes to fabricate a diversity of compounds that affect community stability. Drought encountering soils enriched in antibiotics, which drought-liberal bacteria may construct to suppress other bacteria for existing resources or as signals to enhance drought-encountering mechanisms like biofilm formation (Bouskill et al. 2016a). During drought, bacteria produce compounds that affect rhizosphere soil aggregate stability and hydrophobicity (Kohler et al. 2009). Finally, osmolytes maintain cellular turgor and protect macromolecular structures (Welsh 2000). Season has the biggest influence on the root endosphere microbiome of wild and cultivated Agave species, while host species influence the rhizosphere and leaf phyllosphere (Desgarennes et al. 2014; Coleman-Derr et al. 2016). Cacti and Populusdeltoides showed seasonal root endosphere influence (Shakya et al. 2013; Fonseca-García et al. 2016). In the wildflower genus Banksia (Marschner et al. 2005) and date palms, seasonal soil moisture changes affect the diversity of root and rhizosphere bacterial community (Cherif et al. 2015). The drought regime was the second-biggest beta-diversity factor in cereal grasses after the plant compartment (Naylor et al. 2017). The tree species P. deltoids (Shakya et al. 2013), Mimosa tenuiflora (Taketani et al. 2017), and the cactus Cereus jamacaru have rhizosphere communities that show increased abundance of Actinobacteria, Acidobacteria, and Bacillus in the dry season and Proteobacteria and Bacteroidetes in the rainy season (Nessner Kavamura et al. 2013). Generations of repeated drought events may have evolved stable and beneficial plant-microbe interactions that improve host and microbe reproductive fitness. In one study, Brassica rapa plants exposed to generations of drought were better than controls at increasing bacterial abundance and diversity around roots in dry contemporary environments Because microbes with PGP activities improve plant health and fitness, stable plant-microbiome interactions are an attractive target for crop stress tolerance (Quiza et al. 2015). Plants may select for certain microbial traits by studying drought-treated plant growth-promoting microbes. Rhizosphere isolates had more enzymes than the bulk soil isolates (Timmusk et al. 2011). In a pepper plant study, root endophytes dominated the phyto-hormone synthesis and rhizosphere whereas bulk soil dominated the nutrient solubilizers (Marasco et al. 2012). Only during drought conditions, PGPB can improve drought tolerance as well as plant growth. Bacterial consortia may reduce drought-induced stress better than the individual genera (Knoth et al. 2014; Timm et al. 2016). In a study, microbes have shown the initial positive effects on plant performance but negative effects during severe drought conditions (Redman et al. 2002). Plant physiological responses to severe drought can be greatly influenced by soil microbial communities during well-watered and moderate drought conditions (i.e., zero soil moisture). Soil microbes regulate soil moisture, nutrient availability, and plant function [1, 5]. Azospirillum brasilense increased ABA content in a microbe–ABA interaction study (Cohen et al. 2015). In Oryza sativa, endophytic bacteria increased SA and ABA concentrations, possibly improving stress tolerance (Shahzad et al. 2017). Interestingly, over-expression of certain stress genes has been observed in experimental conditions which elevate the expression of salicylic acid, and regulate plant growth and the immune responses (Devarajan et al. 2021). It was shown that Pseudomonas putida (FBKV2) inoculation increased the SnRK2 family proteins and ABA-responsive gene transcription (SkZ et al. 2018). A treasure of plant and symbiotic microbes is listed in Table 2.
Evident relations of viral pathogenesis and symbiotic bacteria in plants
Stress factors can compromise the plant's immune defenses rendering it susceptible to viruses. However, growth-promoting bacteria enhance plant growth by several factors. The above-mentioned root bacteria help plants against various biotic and abiotic stresses. These beneficial bacteria's are called "plant growth-promoting rhizobacteria" (PGPR). Viruses, on the contrary, were shown to have rare beneficial roles such as conferring resistance against various abiotic stresses. Abiotic stress like drought, salinity, and changing climatic temperatures trigger physiological responses that suppress plant's immune functions offering numerous advantages to opportunistic viruses. Contrarily, symbiotic bacteria alleviate the negative impact of abiotic stress and bolster the plant's immune capacity against infections. PGPRs have been shown to help plants against viral infections by inducing systemic resistance (Yu et al., 2022). It was shown that when plants are exposed to these bacteria, the immune system gets more active and fends off potential pathogens. Zehnder et al., 2000 reported that PGPRs induced systemic resistance (ISR) against Cucumber mosaic cucumovirus infection in tomatoes. Maurhofer et al., 1998 confirmed the expression of salicylic acid biosynthesis genes in Pseudomonas fluorescens strain P3 that induced systemic resistance against Tobacco necrosis virus in tobacco plants. A jasmonic acid-dependent and SA-independent signalling pathway induced by PGPR isolates (Serratia marcescens strain 90-166 and Bacillus pumilus SE34) provides protection against CMV in A. thaliana (Ryu et al., 2004). Hence, PGPRs influence plant hormones level which in turn impact plant's immune responses against viral infections.
PGPRs can also produce certain compounds or enzymes that directly affect or inhibit the growth of viruses. A 39.4 KDa anticiotic protein has been identified and purified from the P. fluorescens strain CZ which has confirmed the 74.81% inhibitory effect against TMV (Shen et al, 2013). Some volatile organic compounds like 2,3-butanediol produced by bacteria P. chlororaphis O6, provided resistance against cucumber mosaic virus, tobacco mosaic virus, pepper mottle virus, and tomato yellow leaf curl virus in pepper (Kong et al., 2018). Application of P. fluorescens controlled Squash mosaic virus infection in cucumber (Firmansyah et al., 2017). Whereas Elbeshehy et al., 2015, showed resistance induction in pumpkins against watermelon mosaic virus by application of B. subtilis and B. pumilus. A large number of defense enzymes like Dicer-like (DCL) enzymes, binases, baRNases, peroxidase and phenylalanine ammonia-lyase etc. are activated by application of PGPRs on plants, inhibiting viruses through inactivation of their RNA or by strengthening the plant’s inborn immunity (Manjunatha et al., 2022). These bacteria mediate modulation of defense enzyme pathways that fine-tune the plant's response to both stress and viruses. The interplay between these factors contributes towards a harmonious defense mechanism that not only protects plants from abiotic stress but also makes plants resilient against viral infections. The research cites the importance and opportunities to harness the potential of root symbiotic bacteria for sustainable agricultural practices, ensuring robust crop protection in the face of changing environmental conditions.
Conclusions and future directions
In conclusion, the present review has elaborated on the pathological outcomes of viral pathogens and beneficial outcomes of symbiotic bacteria in plants. The perspective on such interactions primarily revolves around examining viral pathogenesis under both (abiotic stress and natural environmental) conditions. Simultaneously, discussions have been presented regarding the beneficial role of symbiotic bacteria residing in the plant roots, which directly or indirectly contribute to plant growth promotion and help plants to cope-up with abiotic stress. However, limited literature is available to establish an evident relationship between "viral pathogenesis-abiotic stress-root microbes" conferring the respective harmful and beneficial outcomes, and this part was also discussed precisely. Hence the information gathered in the review is crucial to design future studies that shed light on the intricate association between pathogens, hosts, and symbionts, with a focus on plant/microbial biotechnology and understanding the dynamics of host-microbe relationships to achieve a balanced interplay between plants and bacterial microbes. Furthermore, there is a need for further exploration to better comprehend the role of mycorrhiza and their associated endophytes in enhancing plant immunity against pathogens, as well as their involvement in nutrient acquisition processes from the soil.
Availability of data and materials
Not applicable.
Abbreviations
- ACC oxidase:
-
1-Aminocyclopropane-1-carboxylic acid oxidase
- BIK1:
-
Botrytis-Induced Kinase1
- DOC:
-
Dissolved organic carbon
- EPS:
-
Exopolysaccharides
- ETI:
-
Effectors triggered immunity
- HKT1:
-
High affinity K transporter 1
- HR:
-
Hypersensitive response
- HSPs:
-
Heat shock proteins
- HT:
-
Halotolerant
- MAPK:
-
Mitogen activated protein kinases
- NADP:
-
Nicotinamide adenine dinucleotide phosphate
- NBLRR protein:
-
Nucleotide Binding Domain and Leucine-rich Repeat.
- PCD:
-
Plant cell death
- PGPR:
-
Plant growth-promoting rhizobacteria
- PR genes:
-
Pathogenesis Related genes
- PTI:
-
Pattern triggered immunity
- R-gene:
-
Resistance genes
- ROS:
-
Reactive oxygen species
- siRNAs:
-
Small interfering RNAs
- SMs:
-
Secondary Metabolites
- SOS:
-
Salt overly sensitive
- VOCs:
-
Volatile organic compounds
- vsiRNAs:
-
Viroid derived small interfering RNAs
References
Acosta-Martínez V, Cotton J, Gardner T, Moore-Kucera J, Zak J, Wester D, Cox S (2014) Predominant bacterial and fungal assemblages in agricultural soils during a record drought/heat wave and linkages to enzyme activities of biogeochemical cycling. Appl Soil Ecol 84:69–82. https://doi.org/10.1016/j.apsoil.2014.06.005
Aguilar E, Cutrona C, Del Toro FJ, Vallarino JG, Osorio S, Pérez-Bueno ML, Barón M, Chung BN, Canto T, Tenllado F (2017) Virulence determines beneficial trade-offs in the response of virus-infected plants to drought via induction of salicylic acid. Plant Cell Environ 40:2909–2930. https://doi.org/10.1111/pce.13028
Alfenas-Zerbini P, Maia IG, Fávaro RD, Cascardo JC, Brommonschenkel SH, Zerbini FM (2009) Genome-wide analysis of differentially expressed genes during the early stages of tomato infection by a potyvirus. Mol Plant Microbe Interact 22:352–361. https://doi.org/10.1094/mpmi-22-3-0352
Alster CJ, German DP, Lu Y, Allison SD (2013) Microbial enzymatic responses to drought and to nitrogen addition in a southern California grassland. Soil Biol Biochem 64:68–79. https://doi.org/10.1016/j.soilbio.2013.03.034
Andrews JH, Harris RF (2000) The ecology and biogeography of microorganisms on plant surfaces. Ann Rev Phytopathol 38:145–180. https://doi.org/10.1146/annurev.phyto.38.1.145
Anfoka G, Moshe A, Fridman L, Amrani L, Rotem O, Kolot M, Zeidan M, Czosnek H, Gorovits R (2016) Tomato yellow leaf curl virus infection mitigates the heat stress response of plants grown at high temperatures. Sci Rep 6:19715. https://doi.org/10.1038/srep19715
Arif Y, Singh P, Siddiqui H, Bajguz A, Hayat S (2020) Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol Biochem 156:64–77. https://doi.org/10.1016/j.plaphy.2020.08.042
Armstrong A, Valverde A, Ramond JB, Makhalanyane TP, Jansson JK, Hopkins DW, Aspray TJ, Seely M, Trindade MI, Cowan DA (2016) Temporal dynamics of hot desert microbial communities reveal structural and functional responses to water input. Sci Rep 6:34434. https://doi.org/10.1038/srep34434
Arora NK, Fatima T, Mishra J, Mishra I, Verma S, Verma R, Verma M, Bhattacharya A, Verma P, Mishra P, Bharti C (2020) Halo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J Adv Res 26:69–82. https://doi.org/10.1016/j.jare.2020.07.003
Audil G, Ajaz Ahmad L, Noor Ul Islam W (2019) Biotic and Abiotic Stresses in Plants. In: Alexandre Bosco de O (ed) Abiotic and Biotic Stress in Plants. IntechOpen, Rijeka, p Ch. 1. https://doi.org/10.5772/intechopen.85832
Bachar A, Al-Ashhab A, Soares MI, Sklarz MY, Angel R, Ungar ED, Gillor O (2010) Soil Microbial Abundance and Diversity Along a Low Precipitation Gradient. Microb Ecol 60:453–461. https://doi.org/10.1007/s00248-010-9727-1
Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, Subramanian S, Smith DL (2018) Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 9:1473. https://doi.org/10.3389/fpls.2018.01473
Baebler S, Krecic-Stres H, Rotter A, Kogovsek P, Cankar K, Kok EJ, Gruden K, Kovac M, Zel J, Pompe-Novak M, Ravnikar M (2009) PVY(NTN) elicits a diverse gene expression response in different potato genotypes in the first 12 h after inoculation. Mol Plant Pathol 10:263–275. https://doi.org/10.1111/j.1364-3703.2008.00530.x
Balasooriya WK, Denef K, Huygens D, Boeckx P (2014) Translocation and turnover of rhizodeposit carbon within soil microbial communities of an extensive grassland ecosystem. Plant Soil 376:61–73. https://doi.org/10.1007/s11104-012-1343-z
Barnard RL, Osborne CA, Firestone MK (2013) Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J 7:2229–2241. https://doi.org/10.1038/ismej.2013.104
Baron C, Domke N, Beinhofer M, Hapfelmeier S (2001) Elevated temperature differentially affects virulence, VirB protein accumulation, and T-pilus formation in different Agrobacterium tumefaciens and Agrobacterium vitis strains. J Bacteriol 183:6852–6861. https://doi.org/10.1128/jb.183.23.6852-6861.2001
Bashan Y (1998) Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant-growth-promoting bacteria) and PGPB. Soil Biol Biochem 30:1225–1228. https://doi.org/10.1016/S0038-0717(97)00187-9
Bergès SE, Vile D, Vazquez-Rovere C, Blanc S, Yvon M, Bédiée A, Rolland G, Dauzat M, van Munster M (2018) Interactions Between Drought and Plant Genotype Change Epidemiological Traits of Cauliflower mosaic virus. Front Plant Sci 9:703. https://doi.org/10.3389/fpls.2018.00703
Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A (2016) Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep. 6:34768. https://doi.org/10.1038/srep34768
Bhat MA, Kumar V, Bhat MA, Wani IA, Dar FL, Farooq I, Bhatti F, Koser R, Rahman S, Jan AT (2020) Mechanistic insights of the interaction of plant growth-promoting rhizobacteria (PGPR) with plant roots toward enhancing plant productivity by alleviating salinity stress. Front Microbiol 11:1952. https://doi.org/10.3389/fmicb.2020.01952
Bilal R, Rasul G, Arshad M, Malik K (1993) Attachment, colonization and proliferation of Azospirillum brasilense and Enterobacter spp. on root surface of grasses. World J Microbiol Biotechnol 9:63–69. https://doi.org/10.1007/BF00656519
Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4:273. https://doi.org/10.3389/fpls.2013.00273
Blanc S, Michalakis Y (2016) Manipulation of hosts and vectors by plant viruses and impact of the environment. Curr Opin Insect Sci 16:36–43. https://doi.org/10.1016/j.cois.2016.05.007
Blazewicz SJ, Schwartz E, Firestone MK (2014) Growth and death of bacteria and fungi underlie rainfall-induced carbon dioxide pulses from seasonally dried soil. Ecology 95:1162–1172. https://doi.org/10.1890/13-1031.1
Bolton MD (2009) Primary metabolism and plant defense–fuel for the fire. Mol Plant Microbe Interact 22:487–497. https://doi.org/10.1094/mpmi-22-5-0487
Bouskill NJ, Lim HC, Borglin S, Salve R, Wood TE, Silver WL, Brodie EL (2013) Pre-exposure to drought increases the resistance of tropical forest soil bacterial communities to extended drought. ISME J 7:384–394. https://doi.org/10.1038/ismej.2012.113
Bouskill NJ, Wood TE, Baran R, Hao Z, Ye Z, Bowen BP, Lim HC, Nico PS, Holman HY, Gilbert B, Silver WL, Northen TR, Brodie EL (2016a) Belowground Response to Drought in a Tropical Forest Soil. II. Change in Microbial Function Impacts Carbon Composition. Front Microbiol 7:323. https://doi.org/10.3389/fmicb.2016.00323
Bouskill NJ, Wood TE, Baran R, Ye Z, Bowen BP, Lim H, Zhou J, Nostrand JD, Nico P, Northen TR, Silver WL, Brodie EL (2016b) Belowground Response to Drought in a Tropical Forest Soil. I. Changes in Microbial Functional Potential and Metabolism. Front Microbiol 7:525. https://doi.org/10.3389/fmicb.2016.00525
Calil IP, Fontes EPB (2017) Plant immunity against viruses: antiviral immune receptors in focus. Ann Bot 119:711–723. https://doi.org/10.1093/aob/mcw200
Chen C, Xin K, Liu H, Cheng J, Shen X, Wang Y, Zhang L (2017) Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci Rep 7:41564. https://doi.org/10.1038/srep41564
Cheng C, Gao X, Feng B, Sheen J, Shan L, He P (2013) Plant immune response to pathogens differs with changing temperatures. Nature Commun 4:2530. https://doi.org/10.1038/ncomms3530
Cherif H, Marasco R, Rolli E, Ferjani R, Fusi M, Soussi A, Mapelli F, Blilou I, Borin S, Boudabous A, Cherif A, Daffonchio D, Ouzari H (2015) Oasis desert farming selects environment-specific date palm root endophytic communities and cultivable bacteria that promote resistance to drought. Environ Microbiol Rep 7:668–678. https://doi.org/10.1111/1758-2229.12304
Chung BN, Lee JH, Kang BC, Koh SW, Joa JH, Choi KS, Ahn JJ (2018) HR-Mediated Defense Response is Overcome at High Temperatures in Capsicum Species. Plant Pathol J 34:71–77. https://doi.org/10.5423/ppj.Nt.06.2017.0120
Chung BN, Choi KS, Ahn JJ, Joa JH, Do KS, Park KS (2015) Effects of Temperature on Systemic Infection and Symptom Expression of Turnip mosaic virus in Chinese cabbage (Brassica campestris). Plant Pathol J 31:363–370. https://doi.org/10.5423/ppj.Nt.06.2015.0107
Cohen AC, Bottini R, Pontin M, Berli FJ, Moreno D, Boccanlandro H, Travaglia CN, Piccoli PN (2015) Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol Plant 153:79–90. https://doi.org/10.1111/ppl.12221
Coleman-Derr D, Desgarennes D, Fonseca-Garcia C, Gross S, Clingenpeel S, Woyke T, North G, Visel A, Partida-Martinez LP, Tringe SG (2016) Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol 209:798–811. https://doi.org/10.1111/nph.13697
Connon SA, Lester ED, Shafaat HS, Obenhuber DC, Ponce A (2007) Bacterial diversity in hyperarid Atacama Desert soils. J Geophys Res 112:G04S17. https://doi.org/10.1029/2006JG000311
Corrales-Gutierrez M, Medina-Puche L, Yu Y, Wang L, Ding X, Luna AP, Bejarano ER, Castillo AG, Lozano-Duran R (2020) The C4 protein from the geminivirus Tomato yellow leaf curl virus confers drought tolerance in Arabidopsis through an ABA-independent mechanism. Plant Biotechnol J 18:1121–1123. https://doi.org/10.1111/pbi.13280
Curiel Yuste J, Fernandez-Gonzalez AJ, Fernandez-Lopez M, Ogaya R, Penuelas J, Sardans J, Lloret F (2014) Strong functional stability of soil microbial communities under semiarid Mediterranean conditions and subjected to long-term shifts in baseline precipitation. Soil Biol Biochem 69:223–233. https://doi.org/10.1016/j.soilbio.2013.10.045
Davis TS, Bosque-Pérez NA, Foote NE, Magney T, Eigenbrode SD (2015) Environmentally dependent host–pathogen and vector–pathogen interactions in the Barley yellow dwarf virus pathosystem. J Appl Ecol 52:1392–1401. https://doi.org/10.1111/1365-2664.12484
de la Torre F, Cañas RA, Pascual MB, Avila C, Cánovas FM (2014) Plastidic aspartate aminotransferases and the biosynthesis of essential amino acids in plants. J Exp Bot 65:5527–5534. https://doi.org/10.1093/jxb/eru240
Desaint H, Aoun N, Deslandes L, Vailleau F, Roux F, Berthomé R (2021) Fight hard or die trying: when plants face pathogens under heat stress. New Phytol 229:712–734. https://doi.org/10.1111/nph.16965
Desgarennes D, Garrido E, Torres-Gomez MJ, Peña-Cabriales JJ, Partida-Martinez LP (2014) Diazotrophic potential among bacterial communities associated with wild and cultivated Agave species. FEMS Microbiol Ecol 90:844–857. https://doi.org/10.1111/1574-6941.12438
Devarajan AK, Muthukrishanan G, Truu J, Truu M, Ostonen I, Kizhaeral S S, Panneerselvam P, Kuttalingam Gopalasubramanian S (2021) The Foliar Application of Rice Phyllosphere Bacteria induces Drought-Stress Tolerance in Oryza sativa (L.). Plants (Basel) 10:387. https://doi.org/10.3390/plants10020387
Dodd IC, Jiang F, Teijeiro RG, Belimov AA, Hartung W (2009) The rhizosphere bacterium Variovorax paradoxus 5C–2 containing ACC deaminase does not increase systemic ABA signaling in maize (Zea mays L.). Plant Signal Behav 4:519–521. https://doi.org/10.4161/psb.4.6.8574
Dubey S, Khatri S, Bhattacharjee A, Sharma S (2022) Multiple passaging of rhizospheric microbiome enables mitigation of salinity stress in Vigna radiata. Plant Growth Regul 97:537–549. https://doi.org/10.1007/s10725-022-00820-1
Elbeshehy EK, Youssef SA, Elazzazy AM (2015) Resistance induction in pumpkin Cucurbita maxima L. against Watermelon mosaic potyvirus by plant growth-promoting rhizobacteria. Biocontrol Science and Technology 25:525–542. https://doi.org/10.1080/09583157.2014.994198
El-Esawi MA, Alaraidh IA, Alsahli AA, Alzahrani SM, Ali HM, Alayafi AA, Ahmad M (2018) Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential, ion homeostasis, leaf gas exchange and stress-related gene expression. Int J Mol Sci 19:3310. https://doi.org/10.3390/ijms19113310
Erb M, Kliebenstein DJ (2020) Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol 184:39–52. https://doi.org/10.1104/pp.20.00433
Etesami H (2018) Can interaction between silicon and plant growth promoting rhizobacteria benefit in alleviating abiotic and biotic stresses in crop plants? Agric Ecosyst Environ 253:98–112. https://doi.org/10.1016/j.agee.2017.11.007
Etesami H, Beattie GA (2018) Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front Microbiol 9:148. https://doi.org/10.3389/fmicb.2018.00148
Etesami H, Glick BR (2020) Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Envir Exp Botany 178:104124. https://doi.org/10.1016/j.envexpbot.2020.104124
Falk BW, Nouri S (2020) Special Issue: "Plant Virus Pathogenesis and Disease Control". Viruses 12 https://doi.org/10.3390/v12091049
Farooq M, Hussain M, Wakeel A, Siddique KHM (2015) Salt stress in maize: effects, resistance mechanisms, and management A review. Agron Sustain Dev 35:461–481. https://doi.org/10.1007/s13593-015-0287-0
Fesenko I, Spechenkova N, Mamaeva A, Makhotenko AV, Love AJ, Kalinina NO, Taliansky M (2021) Role of the methionine cycle in the temperature-sensitive responses of potato plants to potato virus Y. Mol Plant Pathol 22:77–91. https://doi.org/10.1111/mpp.13009
Firmansyah D, Hidayat S, Widodo W (2017) Chitosan and Plant Growth PromotingRhizobacteria Application to Control Squash mosaic virus on Cucumber Plants. Asian Journal of Plant Pathology 11:148–155. https://doi.org/10.3923/AJPPAJ.2017.148.155
Food and Agriculture Organization of the United Nation (2022) Global Soil Partnership, Highlight Archives, World Soil Day: FAO highlights the threat of soil salinization to global food security. https://www.fao.org/newsroom/detail/world-soil-day-fao-highlights-threat-of-soilsalinization-to-food-security-031221/en. Accessed 15 June 2023
Fonseca-García C, Coleman-Derr D, Garrido E, Visel A, Tringe SG, Partida-Martínez LP (2016) The Cacti Microbiome: Interplay between Habitat-Filtering and Host-Specificity. Front Microbiol 7:150. https://doi.org/10.3389/fmicb.2016.00150
Franklin J, Serra-Diaz JM, Syphard AD, Regan HM (2016) Global change and terrestrial plant community dynamics. Proc Natl Acad Sci U S A 113:3725–3734. https://doi.org/10.1073/pnas.1519911113
Frey SD, Lee J, Melillo JM, Six J (2013) The temperature response of soil microbial efficiency and its feedback to climate. Nat Clim Change 3:395–398. https://doi.org/10.1038/nclimate1796
Fuchslueger L, Bahn M, Fritz K, Hasibeder R, Richter A (2014) Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow. New Phytol 201:916–927. https://doi.org/10.1111/nph.12569
Gagné-Bourque F, Mayer BF, Charron JB, Vali H, Bertrand A, Jabaji S (2015) Accelerated Growth Rate and Increased Drought Stress Resilience of the Model Grass Brachypodium distachyon Colonized by Bacillus subtilis B26. PLoS One 10:e0130456. https://doi.org/10.1371/journal.pone.0130456
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80. https://doi.org/10.1186/gb-2004-5-10-r80
Gharsallah C, Fakhfakh H, Grubb D, Gorsane F (2016) Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 8 https://doi.org/10.1093/aobpla/plw055
Ghoshal B, Sanfaçon H (2014) Temperature-dependent symptom recovery in Nicotiana benthamiana plants infected with tomato ringspot virus is associated with reduced translation of viral RNA2 and requires ARGONAUTE 1. Virology 456–457:188–197. https://doi.org/10.1016/j.virol.2014.03.026
Goldstein AH (1986) Bacterial solubilization of mineral phosphates: Historical perspective and future prospects. Am J Altern Agric 1:51–57. https://doi.org/10.1017/S0889189300000886
Gong Z, Xiong L, Shi H, Yang S, Herrera-Estrella LR, Xu G, Chao DY, Li J, Wang PY, Qin F, Li J, Ding Y, Shi Y, Wang Y, Yang Y, Guo Y, Zhu JK (2020) Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci 63:635–674. https://doi.org/10.1007/s11427-020-1683-x
Gong Z (2021) Plant abiotic stress: New insights into the factors that activate and modulate plant responses. J Integr Plant Biol 63:429–430. https://doi.org/10.1111/jipb.13079
González R, Butković A, Elena SF (2020) From foes to friends: Viral infections expand the limits of host phenotypic plasticity. Adv Virus Res 106:85–121. https://doi.org/10.1016/bs.aivir.2020.01.003
González R, Butković A, Escaray FJ, Martínez-Latorre J, Melero Í, Pérez-Parets E, Gómez-Cadenas A, Carrasco P, Elena SF (2021) Plant virus evolution under strong drought conditions results in a transition from parasitism to mutualism. Proc Natl Acad Sci U S A 118:e2020990118. https://doi.org/10.1073/pnas.2020990118
Gorovits R, Sobol I, Altaleb M, Czosnek H, Anfoka G (2019) Taking advantage of a pathogen: understanding how a virus alleviates plant stress response. Phytopathol Res 1:1–6. https://doi.org/10.1186/s42483-019-0028-4
Gul S, Javed S, Azeem M, Aftab A, Anwaar N, Mehmood T, Zeshan B (2023) Application of Bacillus subtilis for the Alleviation of Salinity Stress in Different Cultivars of Wheat (Tritium aestivum L.). Agronomy 13:437. https://doi.org/10.3390/agronomy13020437
Guo H, Gu L, Liu F, Chen F, Ge F, Sun Y (2019) Aphid-borne Viral Spread Is Enhanced by Virus-induced Accumulation of Plant Reactive Oxygen Species. Plant Physiol 179:143–155. https://doi.org/10.1104/pp.18.00437
Haldar S, Sengupta S (2015) Plant-microbe Cross-talk in the Rhizosphere: Insight and Biotechnological Potential. Open Microbiol J 9:1–7. https://doi.org/10.2174/1874285801509010001
Haque MM, Biswas MS, Mosharaf MK, Haque MA, Islam MS, Nahar K, Islam MM, Shozib HB, Islam MM, Ferdous-E-Elahi (2022) Halotolerant biofilm-producing rhizobacteria mitigate seawater-induced salt stress and promote growth of tomato. Sci Rep 12:5599. https://doi.org/10.1038/s41598-022-09519-9
Hardoim PR, van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, Döring M, Sessitsch A (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79:293–320. https://doi.org/10.1128/MMBR.00050-14
Hartmann M, Brunner I, Hagedorn F, Bardgett RD, Stierli B, Herzog C, Chen X, Zingg A, Graf-Pannatier E, Rigling A, Frey B (2017) A decade of irrigation transforms the soil microbiome of a semi-arid pine forest. Mol Ecol 26:1190–1206. https://doi.org/10.1111/mec.13995
Hasegawa H, Chatterjee A, Cui Y, Chatterjee A (2005) Elevated temperature enhances virulence of Erwinia carotovora subsp. carotovora strain EC153 to plants and stimulates production of the quorum sensing signal, N-acyl homoserine lactone, and extracellular proteins. Appl Environ Microbiol 71:4655–4663. https://doi.org/10.1128/aem.71.8.4655-4663.2005
Hatfield JL, Prueger JH (2015) Temperature extremes: Effect on plant growth and development. Weather Clim Extremes 10:4–10. https://doi.org/10.1016/j.wace.2015.08.001
He M, He C-Q, Ding N-Z (2018) Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front Plant Sci 9:1771. https://doi.org/10.3389/fpls.2018.01771
Hernández-Walias FJ, García M, Moreno M, Giannoukos I, González N, Sanz-García E, Necira K, Canto T, Tenllado F (2022) Transgenerational tolerance to salt and osmotic stresses induced by plant virus infection. Int J Mol Sci 23:12497. https://doi.org/10.3390/ijms232012497
Horino O, Mew TW, Yamada T (1982) The effect of temperature on the development of bacterial leaf blight on rice. Japanese J Phytopathol 48:72–75. https://doi.org/10.3186/jjphytopath.48.72
Hueso S, García C, Hernández T (2012) Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biol Biochem 50:167–173. https://doi.org/10.1016/j.soilbio.2012.03.026
Hulten E, Jackson JL, Douglas K, George S, Villines TC (2006) The effect of early, intensive statin therapy on acute coronary syndrome: a meta-analysis of randomized controlled trials. Arch Intern Med 166:1814–1821. https://doi.org/10.1001/archinte.166.17.1814
Issa A, Esmaeel Q, Sanchez L, Courteaux B, Guise JF, Gibon Y, Ballias P, Clément C, Jacquard C, Vaillant-Gaveau N, Aït Barka E (2018) Impacts of Paraburkholderia phytofirmans Strain PsJN on Tomato (Lycopersicon esculentum L.) Under High Temperature. Front Plant Sci 9:1397 https://doi.org/10.3389/fpls.2018.01397
Janda M, Lamparová L, Zubíková A, Burketová L, Martinec J, Krčková Z (2019) Temporary heat stress suppresses PAMP-triggered immunity and resistance to bacteria in Arabidopsis thaliana. Mol Plant Pathol 20:1005–1012. https://doi.org/10.1111/mpp.12799
Javed M, Arshad M (1997) Growth promotion of two wheat cultivars by plant growth promoting rhizobacteria. Pak J Bot 29:243–248
Jensen KD, Beier C, Michelsen A, Emmett BA (2003) Effects of experimental drought on microbial processes in two temperate heathlands at contrasting water conditions. Appl Soil Ecol 24:165–176. https://doi.org/10.1016/S0929-1393(03)00091-X
Jin S, Song Y, Deng W, Gordon MP, Nester E (1993) The regulatory VirA protein of Agrobacterium tumefaciens does not function at elevated temperatures. J Bacteriol 175:6830–6835. https://doi.org/10.1128/jb.175.21.6830-6835.1993
Jones LM, Koehler AK, Trnka M, Balek J, Challinor AJ, Atkinson HJ, Urwin PE (2017) Climate change is predicted to alter the current pest status of Globodera pallida and G. rostochiensis in the United Kingdom. Glob Chang Biol 23:4497–4507. https://doi.org/10.1111/gcb.13676
Kang SM, Radhakrishnan R, Khan AL, Kim MJ, Park JM, Kim BR, Shin DH, Lee IJ (2014) Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol Biochem 84:115–124. https://doi.org/10.1016/j.plaphy.2014.09.001
Karhu K, Auffret MD, Dungait JA, Hopkins DW, Prosser JI, Singh BK, Subke JA, Wookey PA, Agren GI, Sebastià MT, Gouriveau F, Bergkvist G, Meir P, Nottingham AT, Salinas N, Hartley IP (2014) Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 513:81–84. https://doi.org/10.1038/nature13604
Khalvandi M, Siosemardeh A, Roohi E, Keramati S (2021) Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 7:e05908. https://doi.org/10.1016/j.heliyon.2021.e05908
Király L, Hafez YM, Fodor J, Király Z (2008) Suppression of tobacco mosaic virus-induced hypersensitive-type necrotization in tobacco at high temperature is associated with downregulation of NADPH oxidase and superoxide and stimulation of dehydroascorbate reductase. J Gen Virol 89:799–808. https://doi.org/10.1099/vir.0.83328-0
Kissoudis C, van de Wiel C, Visser RG, van der Linden G (2014) Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front Plant Sci 5:207. https://doi.org/10.3389/fpls.2014.00207
Kissoudis C, Sunarti S, van de Wiel C, Visser RG, van der Linden CG, Bai Y (2016) Responses to combined abiotic and biotic stress in tomato are governed by stress intensity and resistance mechanism. J Exp Bot 67:5119–5132. https://doi.org/10.1093/jxb/erw285
Knoth JL, Kim S-H, Ettl GJ, Doty SL (2014) Biological nitrogen fixation and biomass accumulation within poplar clones as a result of inoculations with diazotrophic endophyte consortia. New Phytol 201:599–609. https://doi.org/10.1111/nph.12536
Kohler J, Caravaca F, Roldán A (2009) Effect of drought on the stability of rhizosphere soil aggregates of Lactuca sativa grown in a degraded soil inoculated with PGPR and AM fungi. Appl Soil Ecol 42:160–165. https://doi.org/10.1016/j.apsoil.2009.03.007
Kong HG, Shin TS, Kim TH, Ryu CM (2018) Stereoisomers of the Bacterial Volatile Compound 2,3-Butanediol Differently Elicit Systemic Defense Responses of Pepper against Multiple Viruses in theField. Front Plant Sci 9:90. https://doi.org/10.3389/fpls.2018.00090
Kubota K, Ng JC (2016) Lettuce chlorosis virus P23 Suppresses RNA Silencing and Induces Local Necrosis with Increased Severity at Raised Temperatures. Phytopathology 106:653–662. https://doi.org/10.1094/phyto-09-15-0219-r
Kumar P, Choudhary M, Halder T, Prakash NR, Singh V, V VT, Sheoran S, T RK, Longmei N, Rakshit S, Siddique KHM (2022) Salinity stress tolerance and omics approaches: revisiting the progress and achievements in major cereal crops. Heredity (Edinb) 128:497–518. https://doi.org/10.1038/s41437-022-00516-2
Kumar S, Tanti B, Patil BL, Mukherjee SK, Sahoo L (2017) RNAi-derived transgenic resistance to Mungbean yellow mosaic India virus in cowpea. PLoS One 12:e0186786. https://doi.org/10.1371/journal.pone.0186786
Kuske CR, Ticknor LO, Miller ME, Dunbar JM, Davis JA, Barns SM, Belnap J (2002) Comparison of soil bacterial communities in rhizospheres of three plant species and the interspaces in an arid grassland. Appl Environ Microbiol 68:1854–1863. https://doi.org/10.1128/aem.68.4.1854-1863.2002
Li Z, Liu H, Ding Z, Yan J, Yu H, Pan R, Hu J, Guan Y, Hua J (2020) Low Temperature Enhances Plant Immunity via Salicylic Acid Pathway Genes That Are Repressed by Ethylene. Plant Physiol 182:626–639. https://doi.org/10.1104/pp.19.01130
Lichtfouse E (2009) Climate change, intercropping, pest control and beneficial microorganisms. Springer. https://doi.org/10.1007/978-90-481-2716-0
Liptzin D, Silver WL, Detto M (2011) Temporal Dynamics in Soil Oxygen and Greenhouse Gases in Two Humid Tropical Forests. Ecosystems 14:171–182. https://doi.org/10.1007/s10021-010-9402-x
Liu J, Feng L, Gu X, Deng X, Qiu Q, Li Q, Zhang Y, Wang M, Deng Y, Wang E, He Y, Bäurle I, Li J, Cao X, He Z (2019) An H3K27me3 demethylase-HSFA2 regulatory loop orchestrates transgenerational thermomemory in Arabidopsis. Cell Res 29:379–390. https://doi.org/10.1038/s41422-019-0145-8
Liu X, Le Roux X, Salles JF (2022) The legacy of microbial inoculants in agroecosystems and potential for tackling climate change challenges. iScience 25:103821. https://doi.org/10.1016/j.isci.2022.103821
Llamas-Llamas ME, Zavaleta-Mejia E, Gonzalez-Hernandez VA, Cervantes-Diaz L, Santizo-Rincon JA, Ochoa-Martinez DL (1998) Effect of temperature on symptom expression and accumulation of tomato spotted wilt virus in different host species. Plant Pathol 47:341–347. https://doi.org/10.1046/j.1365-3059.1998.00249.x
Ma Y, Dias MC, Freitas H (2020) Drought and Salinity Stress Responses and Microbe-Induced Tolerance in Plants. Front Plant Sci 11:591911. https://doi.org/10.3389/fpls.2020.591911
Mahawar L, Shekhawat GS (2019) EsHO 1 mediated mitigation of NaCl induced oxidative stress and correlation between ROS, antioxidants and HO 1 in seedlings of Eruca sativa: underutilized oil yielding crop of arid region. Physiol Mol Biol Plants 25:895–904. https://doi.org/10.1007/s12298-019-00663-7
Mahmood S, Daur I, Al-Solaimani SG, Ahmad S, Madkour MH, Yasir M, Hirt H, Ali S, Ali Z (2016) Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front Plant Sci 7:876. https://doi.org/10.3389/fpls.2016.00876
Makarova S, Makhotenko A, Spechenkova N, Love AJ, Kalinina NO, Taliansky M (2018) Interactive Responses of Potato (Solanum tuberosum L.) Plants to Heat Stress and Infection With Potato Virus Y. Front Microbiol 9:2582 https://doi.org/10.3389/fmicb.2018.02582
Manjunatha L, Rajashekara H, Uppala LS, Ambika DS, Patil B, Shankarappa KS, Nath VS, Kavitha TR, Mishra AK (2022) Mechanisms of Microbial Plant Protection and Control of Plant Viruses. Plants (Basel). 11(24):3449. https://doi.org/10.3390%2Fplants11243449
Marasco R, Rolli E, Ettoumi B, Vigani G, Mapelli F, Borin S, Abou-Hadid AF, El-Behairy UA, Sorlini C, Cherif A, Zocchi G, Daffonchio D (2012) A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS One 7:e48479. https://doi.org/10.1371/journal.pone.0048479
Markham KK, Greenham K (2021) Abiotic stress through time. New Phytol 231:40–46. https://doi.org/10.1111/nph.17367
Márquez LM, Redman RS, Rodriguez RJ, Roossinck MJ (2007) A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science 315:513–515. https://doi.org/10.1126/science.1136237
Marschner P, Grierson PF, Rengel Z (2005) Microbial community composition and functioning in the rhizosphere of three Banksia species in native woodland in Western Australia. Appl Soil Ecol 28:191–201. https://doi.org/10.1016/j.apsoil.2004.09.001
Martiny JB, Martiny AC, Weihe C, Lu Y, Berlemont R, Brodie EL, Goulden ML, Treseder KK, Allison SD (2017) Microbial legacies alter decomposition in response to simulated global change. ISME J 11:490–499. https://doi.org/10.1038/ismej.2016.122
Marulanda A, Azcón R, Chaumont F, Ruiz-Lozano JM, Aroca R (2010) Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 232:533–543. https://doi.org/10.1007/s00425-010-1196-8
Maurhofer M, Reimmann C, Schmidli-Sacherer P, Heeb S, Haas D, Défago G, (1998) Salicylic Acid Biosynthetic Genes Expressed in Pseudomonasfluorescens Strain P3 Improve the Induction of Systemic Resistance in Tobacco AgainstTobacco Necrosis Virus. Phytopathology 88(7):678–84. https://doi.org/10.1094/PHYTO.1998.88.7.678
Mishra J, Arora NK (2018) Secondary metabolites of fluorescent pseudomonads in biocontrol of phytopathogens for sustainable agriculture. Appl Soil Ecol 125:35–45. https://doi.org/10.1016/j.apsoil.2017.12.004
Mishra S, Sahu PK, Agarwal V, Singh N (2021) Exploiting endophytic microbes as micro-factories for plant secondary metabolite production. Appl Microbiol Biotechnol 105:6579–6596. https://doi.org/10.1007/s00253-021-11527-0
Mishra R, Shteinberg M, Shkolnik D, Anfoka G, Czosnek H, Gorovits R (2022) Interplay between abiotic (drought) and biotic (virus) stresses in tomato plants. Mol Plant Pathol 23:475–488. https://doi.org/10.1111/mpp.13172
Mohammadipanah F, Wink J (2016) Actinobacteria from Arid and Desert Habitats: Diversity and Biological Activity. Front Microbiol 6:1541. https://doi.org/10.3389/fmicb.2015.01541
Moldakimova N, Mukiyanova G, Yarmolinsky D, Brychkova G, Scholthof H, Sagi M, Omarov R (2012) Effect of salinity on viral disease spread in plants. J Stress Physiol Biochem 8:S17
Moshe A, Gorovits R, Liu Y, Czosnek H (2016) Tomato plant cell death induced by inhibition of HSP90 is alleviated by Tomato yellow leaf curl virus infection. Mol Plant Pathol 17:247–260. https://doi.org/10.1111/mpp.12275
Moury B, Selassie KG, Marchoux G, Daubèze A-M, Palloix A (1998) High temperature effects on hypersensitive resistance to tomato spotted wilt tospovirus (TSWV) in pepper (Capsicum chinense Jacq.). Eur J Plant Pathol 104:489–498. https://doi.org/10.1023/A:1008618022144
Nadeem SM, Zahir ZA, Naveed M, Arshad M (2009) Rhizobacteria containing ACC deaminase confer salt tolerance in maize grown on salt-affected fields. Can J Microbiol. 55(11):1302–1309. https://doi.org/10.1139/w09-092
Nancarrow N, Constable FE, Finlay KJ, Freeman AJ, Rodoni BC, Trebicki P, Vassiliadis S, Yen AL, Luck JE (2014) The effect of elevated temperature on Barley yellow dwarf virus-PAV in wheat. Virus Res 186:97–103. https://doi.org/10.1016/j.virusres.2013.12.023
Naylor D, DeGraaf S, Purdom E, Coleman-Derr D (2017) Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J 11:2691–2704. https://doi.org/10.1038/ismej.2017.118
Nepomoceno TAR, Carniatto I (2023) Correlations between climate resilience in family farming and sustainable rural development. Ambio:1-15 https://doi.org/10.1007/s13280-023-01848-x
Nessner Kavamura V, Taketani RG, Lançoni MD, Andreote FD, Mendes R, Soares de Melo I (2013) Water regime influences bulk soil and Rhizosphere of Cereus jamacaru bacterial communities in the Brazilian Caatinga biome. PLoS One 8:e73606. https://doi.org/10.1371/journal.pone.0073606
Newsham K, Fitter A, Watkinson A (1995) Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol Evol 10:407–411. https://doi.org/10.1016/s0169-5347(00)89157-0
Niu SQ, Li HR, Paré PW, Aziz M, Wang SM, Shi H, Li J, Han QQ, Guo SQ, Li J, Guo Q, Ma Q, Zhang JL (2016) Induced growth promotion and higher salt tolerance in the halophyte grass Puccinellia tenuiflora by beneficial rhizobacteria. Plant Soil 407:217–230. https://doi.org/10.1007/s11104-015-2767-z
Obrępalska-Stęplowska A, Renaut J, Planchon S, Przybylska A, Wieczorek P, Barylski J, Palukaitis P (2015) Effect of temperature on the pathogenesis, accumulation of viral and satellite RNAs and on plant proteome in peanut stunt virus and satellite RNA-infected plants. Front Plant Sci 6:903. https://doi.org/10.3389/fpls.2015.00903
Park HJ, Kim WY, Yun DJ (2016) A New Insight of Salt Stress Signaling in Plant. Mol Cells 39:447–459. https://doi.org/10.14348/molcells.2016.0083
Park YG, Mun BG, Kang SM, Hussain A, Shahzad R, Seo CW, Kim AY, Lee SU, Oh KY, Lee DY, Lee IJ, Yun BW (2017) Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS One 12:e0173203. https://doi.org/10.1371/journal.pone.0173203
Patel T, Saraf M (2017) Biosynthesis of phytohormones from novel rhizobacterial isolates and their in vitro plant growth-promoting efficacy. J Plant Interact 12:480–487. https://doi.org/10.1080/17429145.2017.1392625
Pillay V, Nowak J (1997) Inoculum density, temperature, and genotype effects on in vitro growth promotion and epiphytic and endophytic colonization of tomato (Lycopersicon esculentum L.) seedlings inoculated with a pseudomonad bacterium. Can J Microbiol 43:354–361. https://doi.org/10.1139/m97-049
Prasad A, Sett S, Prasad M (2022) Plant-virus-abiotic stress interactions in plants: a complex interplay. Environ Exp Botany 199:104869. https://doi.org/10.1016/j.envexpbot.2022.104869
Prasad A, Sharma N, Muthamilarasan M, Rana S, Prasad M (2019a) Recent advances in small RNA mediated plant-virus interactions. Crit Rev Biotechnol 39:587–601. https://doi.org/10.1080/07388551.2019.1597830
Prasad M, Srinivasan R, Chaudhary M, Choudhary M, Jat LK (2019b) Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: perspectives and challenges. PGPR amelioration in sustainable agriculture:129-157. https://doi.org/10.1016/B978-0-12-815879-1.00007-0
Prasch CM, Sonnewald U (2013) Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol 162:1849–1866. https://doi.org/10.1104/pp.113.221044
Quiza L, St-Arnaud M, Yergeau E (2015) Harnessing phytomicrobiome signaling for rhizosphere microbiome engineering. Front Plant Sci 6:507. https://doi.org/10.3389/fpls.2015.00507
Ramegowda V, Senthil-Kumar M, Ishiga Y, Kaundal A, Udayakumar M, Mysore KS (2013) Drought stress acclimation imparts tolerance to Sclerotinia sclerotiorum and Pseudomonas syringae in Nicotiana benthamiana. Int J Mol Sci 14:9497–9513. https://doi.org/10.3390/ijms14059497
Ray S, Casteel CL (2022) Effector-mediated plant-virus-vector interactions. Plant Cell 34:1514–1531. https://doi.org/10.1093/plcell/koac058
Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM (2002) Thermotolerance generated by plant/fungal symbiosis. Science 298:1581. https://doi.org/10.1126/science.1072191
Roberts KE, Hadfield JD, Sharma MD, Longdon B (2018) Changes in temperature alter the potential outcomes of virus host shifts. PLoS Pathog 14:e1007185. https://doi.org/10.1371/journal.ppat.1007185
Rubio L, Galipienso L, Ferriol I (2020) Detection of plant viruses and disease management: Relevance of genetic diversity and evolution. Front Plant Sci 11:1092. https://doi.org/10.3389/fpls.2020.01092
Ryu CM, Murphy JF, Mysore KS, Kloepper JW (2004) Plant growth-promoting rhizobacteriasystemically protect Arabidopsis thaliana against Cucumber mosaic virus by a salicylic acid andNPR1-independent and jasmonic acid-dependent signaling pathway. Plant J. 39:381–392. https://doi.org/10.1111/j.1365-313x.2004.02142.x
Saghafi D, Ghorbanpour M, Shirafkan Ajirloo H, Asgari Lajayer B (2019) Enhancement of growth and salt tolerance in Brassica napus L. seedlings by halotolerant Rhizobium strains containing ACC-deaminase activity. Plant Physiol Rep 24:225–235. https://doi.org/10.1007/s40502-019-00444-0
Sahu PP, Rai NK, Chakraborty S, Singh M, Chandrappa PH, Ramesh B, Chattopadhyay D, Prasad M (2010) Tomato cultivar tolerant to Tomato leaf curl New Delhi virus infection induces virus-specific short interfering RNA accumulation and defence-associated host gene expression. Mol Plant Pathol 11:531–544. https://doi.org/10.1111/j.1364-3703.2010.00630.x
Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B (2010) Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Reg 62:21–30. https://doi.org/10.1007/s10725-010-9479-4
Scholthof KB, Adkins S, Czosnek H, Palukaitis P, Jacquot E, Hohn T, Hohn B, Saunders K, Candresse T, Ahlquist P, Hemenway C, Foster GD (2011) Top 10 plant viruses in molecular plant pathology. Mol Plant Pathol 12:938–954. https://doi.org/10.1111/j.1364-3703.2011.00752.x
Schwartz E, Adair KL, Schuur EA (2007) Bacterial community structure correlates with decomposition parameters along a Hawaiian precipitation gradient. Soil Biol Biochem 39:2164–2167. https://doi.org/10.1016/j.soilbio.2007.02.013
Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, Dindaroglu T, Abdul-Wajid HH, Battaglia ML (2021) Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants (Basel) 10:259. https://doi.org/10.3390/plants10020259
Shahzad R, Khan AL, Bilal S, Waqas M, Kang S-M, Lee I-J (2017) Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ Exp Botany 136:68–77. https://doi.org/10.1016/j.envexpbot.2017.01.010
Shakya M, Gottel N, Castro H, Yang ZK, Gunter L, Labbé J, Muchero W, Bonito G, Vilgalys R, Tuskan G, Podar M, Schadt CW (2013) A multifactor analysis of fungal and bacterial community structure in the root microbiome of mature Populus deltoides trees. PLoS One 8:e76382. https://doi.org/10.1371/journal.pone.0076382
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2:587. https://doi.org/10.1186/2193-1801-2-587
Shen L, Wang F, Yang J, Qian Y, Dong X, Zhan H (2013) Control of tobacco mosaic virus by Pseudomonas fluorescens CZ powder in green houses and the field. Crop Prot 56:87–90
Shokri D, Emtiazi G (2010) Indole-3-acetic acid (IAA) production in symbiotic and non-symbiotic nitrogen-fixing bacteria and its optimization by Taguchi design. Curr Microbiol 61:217–225. https://doi.org/10.1007/s00284-010-9600-y
Shteinberg M, Mishra R, Anfoka G, Altaleb M, Brotman Y, Moshelion M, Gorovits R, Czosnek H (2021) Tomato Yellow Leaf Curl Virus (TYLCV) Promotes Plant Tolerance to Drought. Cells 10:2875. https://doi.org/10.3390/cells10112875
Singh A, Permar V, Basavaraj Tomar BS, Praveen S (2018) Effect of Temperature on Symptoms Expression and Viral RNA Accumulation in Groundnut Bud Necrosis Virus Infected Vigna unguiculata. Iran J Biotechnol 16:e1846. https://doi.org/10.15171/ijb.1846
Sinha R, Gupta A, Senthil-Kumar M (2016) Understanding the Impact of Drought on Foliar and Xylem Invading Bacterial Pathogen Stress in Chickpea. Front Plant Sci 7:902. https://doi.org/10.3389/fpls.2016.00902
Sinha KV, Das SS, Sanan-Mishra N (2021) Overexpression of a RNA silencing suppressor, B2 protein encoded by Flock House virus, in tobacco plants results in tolerance to salt stress. Phytoparasitica 49:299–316. https://doi.org/10.1007/S12600-020-00847-Y
SkZ A, Vardharajula S, Vurukonda SSKP (2018) Transcriptomic profiling of maize (Zea mays L.) seedlings in response to Pseudomonas putida stain FBKV2 inoculation under drought stress. Ann Microbiol 68:331–349. https://doi.org/10.1007/s13213-018-1341-3
Srivastav AL, Dhyani R, Ranjan M, Madhav S, Sillanpää M (2021) Climate-resilient strategies for sustainable management of water resources and agriculture. Environ Sci Pollut Res Int 28:41576–41595. https://doi.org/10.1007/s11356-021-14332-4
Sunita K, Mishra I, Mishra J, Prakash J, Arora NK (2020) Secondary metabolites from halotolerant plant growth promoting rhizobacteria for ameliorating salinity stress in plants. Front Microbiol 11:567768. https://doi.org/10.3389/fmicb.2020.567768
Suntio T, Mäkinen K (2012) Abiotic stress responses promote Potato virus A infection in Nicotiana benthamiana. Mol Plant Pathol 13:775–784. https://doi.org/10.1111/j.1364-3703.2012.00786.x
Tabassum B, Khan A, Tariq M, Ramzan M, Khan MSI, Shahid N, Aaliya K (2017) Bottlenecks in commercialisation and future prospects of PGPR. Appl Soil Ecol 121:102–117. https://doi.org/10.1016/j.apsoil.2017.09.030
Taketani RG, Lançoni MD, Kavamura VN, Durrer A, Andreote FD, Melo IS (2017) Dry Season Constrains Bacterial Phylogenetic Diversity in a Semi-Arid Rhizosphere System. Microbial Ecol 73:153–161. https://doi.org/10.1007/s00248-016-0835-4
Tatineni S, Afunian MR, Hilf ME, Gowda S, Dawson WO, Garnsey SM (2009) Molecular characterization of Citrus tatter leaf virus historically associated with Meyer lemon trees: complete genome sequence and development of biologically active in vitro transcripts. Phytopathology 99:423–431. https://doi.org/10.1094/phyto-99-4-0423
Timm CM, Pelletier DA, Jawdy SS, Gunter LE, Henning JA, Engle N, Aufrecht J, Gee E, Nookaew I, Yang Z, Lu TY, Tschaplinski TJ, Doktycz MJ, Tuskan GA, Weston DJ (2016) Two Poplar-Associated Bacterial Isolates Induce Additive Favorable Responses in a Constructed Plant-Microbiome System. Front Plant Sci 7:497. https://doi.org/10.3389/fpls.2016.00497
Timmusk S, Paalme V, Pavlicek T, Bergquist J, Vangala A, Danilas T, Nevo E (2011) Bacterial distribution in the rhizosphere of wild barley under contrasting microclimates. PLoS One 6:e17968. https://doi.org/10.1371/journal.pone.0017968
Tiwari S, Singh P, Tiwari R, Meena KK, Yandigeri M, Singh DP, Arora DK (2011) Salt-tolerant rhizobacteria-mediated induced tolerance in wheat (Triticum aestivum) and chemical diversity in rhizosphere enhance plant growth. Biol Fertil Soils 47:907–916. https://doi.org/10.1007/s00374-011-0598-5
Travella S, Klimm TE, Keller B (2006) RNA interference-based gene silencing as an efficient tool for functional genomics in hexaploid bread wheat. Plant Physiol 142:6–20. https://doi.org/10.1104/pp.106.084517
Treseder KK, Kivlin SN, Hawkes CV (2011) Evolutionary trade-offs among decomposers determine responses to nitrogen enrichment. Ecol Lett 14:933–938. https://doi.org/10.1111/j.1461-0248.2011.01650.x
Tsai W-A, Shafiei-Peters JR, Mitter N, Dietzgen RG (2022) Effects of Elevated Temperature on the Susceptibility of Capsicum Plants to Capsicum Chlorosis Virus Infection. Pathogens 11:200. https://doi.org/10.3390/pathogens11020200
Tuttle JR, Idris AM, Brown JK, Haigler CH, Robertson D (2008) Geminivirus-mediated gene silencing from Cotton leaf crumple virus is enhanced by low temperature in cotton. Plant Physiol 148:41–50. https://doi.org/10.1104/pp.108.123869
Ullah S, Bano A (2015) Isolation of plant-growth-promoting rhizobacteria from rhizospheric soil of halophytes and their impact on maize (Zea mays L.) under induced soil salinity. Can J Microbiol 61:307–313. https://doi.org/10.1139/cjm-2014-0668
Upadhyay SK, Singh DP (2015) Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol (Stuttg) 17:288–293. https://doi.org/10.1111/plb.12173
Uzma M, Iqbal A, Hasnain S (2022) Drought tolerance induction and growth promotion by indole acetic acid producing Pseudomonas aeruginosa in Vigna radiata. PLoS One 17:e0262932. https://doi.org/10.1371/journal.pone.0262932
van Munster M (2020) Impact of Abiotic Stresses on Plant Virus Transmission by Aphids. Viruses 12:216. https://doi.org/10.3390/v12020216
van Zelm E, Zhang Y, Testerink C (2020) Salt Tolerance Mechanisms of Plants. Annu Rev Plant Biol 71:403–433. https://doi.org/10.1146/annurev-arplant-050718-100005
Varela ALN, Oliveira JTA, Komatsu S, Silva RGG, Martins TF, Souza PFN, Lobo AKM, Vasconcelos IM, Carvalho FEL, Silveira JAG (2019) A resistant cowpea (Vigna unguiculata [L.] Walp.) genotype became susceptible to cowpea severe mosaic virus (CPSMV) after exposure to salt stress. J Proteomics 194:200–217. https://doi.org/10.1016/j.jprot.2018.11.015
Vargas L, Santa Brígida AB, Mota Filho JP, de Carvalho TG, Rojas CA, Vaneechoutte D, Van Bel M, Farrinelli L, Ferreira PC, Vandepoele K, Hemerly AS (2014) Drought tolerance conferred to sugarcane by association with Gluconacetobacter diazotrophicus: a transcriptomic view of hormone pathways. PLoS One 9:e114744. https://doi.org/10.1371/journal.pone.0114744
Velásquez AC, Castroverde CDM, He SY (2018) Plant-Pathogen Warfare under Changing Climate Conditions. Curr Biol 28:R619-r634. https://doi.org/10.1016/j.cub.2018.03.054
Vigani G, Rolli E, Marasco R, Dell'Orto M, Michoud G, Soussi A, Raddadi N, Borin S, Sorlini C, Zocchi G, Daffonchio D (2018) Root bacterial endophytes confer drought resistance and enhance expression and activity of a vacuolar H(+) -pumping pyrophosphatase in pepper plants. Environ Microbiol 21:3212-3228. https://doi.org/10.1111/1462-2920.14272
Volkening JD, Stecker KE, Sussman MR (2019) Proteome-wide Analysis of Protein Thermal Stability in the Model Higher Plant Arabidopsis thaliana. Mol Cell Proteomics 18:308–319. https://doi.org/10.1074/mcp.RA118.001124
Waldon HB, Jenkins MB, Virginia RA, Harding EE (1989) Characteristics of woodland rhizobial populations from surface-and deep-soil environments of the Sonoran Desert. Appl Environ Microbiol 55:3058–3064. https://doi.org/10.1128/aem.55.12.3058-3064.1989
Wang Y, Bao Z, Zhu Y, Hua J (2009) Analysis of temperature modulation of plant defense against biotrophic microbes. Mol Plant Microbe Interact 22:498–506. https://doi.org/10.1094/mpmi-22-5-0498
Wang L, Jin P, Wang J, Jiang L, Shan T, Zheng Y (2015) Methyl jasmonate primed defense responses against Penicillium expansum in sweet cherry fruit. Plant Mol Biol Rep 33:1464–1471. https://doi.org/10.1007/s11105-014-0844-8
Wang S, Liang D, Li C, Hao Y, Ma F, Shu H (2012) Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol Biochem 51:81–89. https://doi.org/10.1016/j.plaphy.2011.10.014
Welsh DT (2000) Ecological significance of compatible solute accumulation by micro-organisms: from single cells to global climate. FEMS Microbiol Rev 24:263–290. https://doi.org/10.1111/j.1574-6976.2000.tb00542.x
Westwood JH, McCann L, Naish M, Dixon H, Murphy AM, Stancombe MA, Bennett MH, Powell G, Webb AA, Carr JP (2013) A viral RNA silencing suppressor interferes with abscisic acid-mediated signalling and induces drought tolerance in Arabidopsis thaliana. Mol Plant Pathol 14:158–170. https://doi.org/10.1111/j.1364-3703.2012.00840.x
Xu Z, Zhou G, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5:649–654. https://doi.org/10.4161/psb.5.6.11398
Xu P, Chen F, Mannas JP, Feldman T, Sumner LW, Roossinck MJ (2008) Virus infection improves drought tolerance. New Phytol 180:911–921. https://doi.org/10.1111/j.1469-8137.2008.02627.x
Xu J, Li XL, Luo L (2012) Effects of engineered Sinorhizobium meliloti on cytokinin synthesis and tolerance of alfalfa to extreme drought stress. Appl Environ Microbiol 78:8056–8061. https://doi.org/10.1128/aem.01276-12
Yang J, Kloepper JW, Ryu C-M (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4. https://doi.org/10.1016/j.tplants.2008.10.004
Yasin NA, Akram W, Khan WU, Ahmad SR, Ahmad A, Ali A (2018) Halotolerant plant-growth promoting rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth in Capsicum annum L. Environ Sci Pollut Res Int 25:23236–23250. https://doi.org/10.1007/s11356-018-2381-8
Yu Y, Gui Y, Li Z, Jiang C, Guo J, Niu D (2022) Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants (Basel) 11(3):386. https://doi.org/10.3390/plants11030386
Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B, Johnson DM, Swift GB, He Y, Siedow JN, Pei ZM (2014) OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514:367–371. https://doi.org/10.1038/nature13593
Zehnder GW, Yao C, Murphy JF, Sikora ER, Kloepper JW (2000) Induction of resistance in tomato against cucumber mosaic cucumovirus by plant growth-promoting rhizobacteria. BioControl 45(1):127–137. https://doi.org/10.1023/A%3A1009923702103
Zhang X, Liu P, Qing C, Yang C, Shen Y, Ma L (2021) Comparative transcriptome analyses of maize seedling root responses to salt stress. PeerJ 9:e10765. https://doi.org/10.7717/peerj.10765
Zhang JL, Aziz M, Qiao Y, Han QQ, Li J, Wang YQ, Shen X, Wang SM, Paré PW (2014) Soil microbe Bacillus subtilis (GB03) induces biomass accumulation and salt tolerance with lower sodium accumulation in wheat. Crop Pasture Sci 65:423–427. https://doi.org/10.1071/CP13456
Zhang X, Zhang X, Singh J, Li D, Qu F (2012) Temperature-dependent survival of Turnip crinkle virus-infected arabidopsis plants relies on an RNA silencing-based defense that requires dcl2, AGO2, and HEN1. J Virol 86:6847–6854. https://doi.org/10.1128/jvi.00497-12
Zhang S, Wu QR, Liu LL, Zhang HM, Gao JW, Pei ZM (2020) Osmotic stress alters circadian cytosolic Ca2+ oscillations and OSCA1 is required in circadian gated stress adaptation. Plant Signal Behav 15:1836883. https://doi.org/10.1080/15592324.2020.1836883
Zhao C, Zhang H, Song C, Zhu J-K, Shabala S (2020) Mechanisms of plant responses and adaptation to soil salinity. Innovation (Camb) 1:100017. https://doi.org/10.1016/j.xinn.2020.100017
Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P (2021) Regulation of Plant Responses to Salt Stress. Int J Mol Sci 22 https://doi.org/10.3390/ijms22094609
Zhao Q, Chen W, Bian J, Xie H, Li Y, Xu C, Ma J, Guo S, Chen J, Cai X, Wang X, Wang Q, She Y, Chen S, Zhou Z, Dai S (2018) Proteomics and Phosphoproteomics of Heat Stress-Responsive Mechanisms in Spinach. Front Plant Sci 9:800. https://doi.org/10.3389/fpls.2018.00800
Zhou Y, Frey TK, Yang JJ (2009) Viral calciomics: interplays between Ca2+ and virus. Cell Calcium 46:1–17. https://doi.org/10.1016/j.ceca.2009.05.005
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273. https://doi.org/10.1146/annurev.arplant.53.091401.143329
Zhu Y, Qian W, Hua J (2010) Temperature modulates plant defense responses through NB-LRR proteins. PLoS Pathog 6:e1000844. https://doi.org/10.1371/journal.ppat.1000844
Zhu F, Qu L, Hong X, Sun X (2011) Isolation and characterization of a phosphate-solubilizing halophilic bacterium Kushneria sp YCWA18 from Daqiao Saltern on the coast of Yellow Sea of China. Evid Based Complement Alternat Med 2011:615032. https://doi.org/10.1155/2011/615032
Acknowledgements
Authors are thankful to Maharishi Markandeshwar Deemed to be University for providing the infrastructure.
Funding
This research received external funding from project EEQ/2021/000312 from Science and Engineering Research Board, DST, Govt. of India. S.D. also acknowledge Department of Biotechnology, Govt. of India for providing funding through EMR project BT/PR40936/AGIII/103/1255/2020.
Author information
Authors and Affiliations
Contributions
Writing- original draft preparation, B.S., S.D., Y.W.D., writing- review and editing V.S., S.A.M., N.D., G.V., H.S.T., A.K.S., Y.W.D., S.D., B.S., supervision- B.S., S.D. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
B.S., S.D., Y.W.D agree for publication.
Competing interests
None.
Additional information
Handling Editor: Dr. Qingmei Guan.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharma, V., Mohammed, S.A., Devi, N. et al. Unveiling the dynamic relationship of viruses and/or symbiotic bacteria with plant resilience in abiotic stress. Stress Biology 4, 10 (2024). https://doi.org/10.1007/s44154-023-00126-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-023-00126-w