Abstract
Seeds of Moringa oleifera were planted in sandy and vermicular soil (1/2 w/w) and irrigated with Hoagland solution. Two weeks later, the seedlings were divided into three groups: The first group was irrigated with a control solution (Hoagland solution), the second with a salt solution (10 g/l NaCl) and the third group with a mixed solution (10 g/l NaCl and 100 mM citric acid (CA)). Salinity induced a substantial inhibitory effect on seedling growth of Moringa oleifera. Salt treatment reduced shoot fresh weight (FW), the content of photosynthetic pigments and total soluble proteins (SP). Furthermore, salt treatment resulted in accumulation of total free amino acids, soluble sugars and proline. CA supply in saline solution improved shoot growth, and photosynthetic pigment and soluble proteins levels. The important content of citric acid in leaves was accompanied with a decrease of total free amino acids, soluble sugar and proline contents. More that, exogenous application of citric acid led to a decrease of Malondialdehyde (MDA) and hydrogen peroxide (H2O2) accumulation, reflecting the reduction in the imbalance situation and membrane damage induced by salt stress. Alternatively, citric acid supply in saline conditions reduced the rise of superoxidase dismutase (SOD) and catalase (CAT) activities induced under salinity. Citric acid enhanced the growth rate of salt-treated Moringa oleifera via enhancing antioxidant function. Consequently, citric acid treatment may be a promising method for improving Moringa oleifera plants’ tolerance to salt stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plant morphology, plant physiology and the biochemical activities of plants are influenced by the current climate change-induced imbalance of temperature, light intensity, soil water retention capacity and other environmental parameters. Specifically, salinity exerts a significant influence on the growth of plants and crops, posing a hazard to global food security [1]. There are 120 nations worldwide with a total area of 954 million hectares that are impacted by salinity [1]. Multiple studies have examined the detrimental effects of salt stress on plants, which include the inhibition of photosynthesis, disruption of ion balance, and peroxidation of cell membranes [2,3,4]. Given the global issue of soil salinization, it is of utmost importance to understand the processes of plant salt tolerance for the purpose of enhancing crop production and developing innovative agricultural practices [4]. Improving salt tolerance in agricultural crops is critical in order to meet the future demands of global food security [1, 5, 6]. Currently, classical breeding and selection; seed priming; wide crossing; transgenic and genome editing are all methods used for improving crop salt tolerance. Several compounds were used for seed priming or as co-treatment to enhance plant growth in salty environments including biochar, salicylic acid and citric acid [7,8,9,10,11].
The Moringa species has attracted a great deal of interest in recent years. Indeed, Moringa oleifera is renowned for its considerable medicinal properties and its exceptional nutritional value [12]. There are currently several attempts to grow Moringa in regions with moderate salinity. Although, moderate salinity improves the antioxidant capacity and regulates the physio-biochemical reactions of Moringa [13. Further research is needed to better understand the effects of salt on the growth of Moringa and especially on the physio-biochemical properties. This is particularly because of the medicinal virtues and values this tree. In order to mitigate salt effect, several studies examine the potential role of a large biomolecules or compounds in tolerance enhancement to abiotic stress. Citing for example Fahed et al. [14] who studied the role of phytohormones (indole acetic acid, abscisic acid, cytokinins, salicylic acid, gibberellic acid, ethylene…) on plant responses to abiotic stresses factors. In another hand, other researchers [15] have showed that salicylic acid mediated increase membrane stability leading to promoting more stress tolerance in Moringa.
CA is a vital component of the Krebs cycle also named citric acid cycle. Krebs cycle is a generator of precursors such as NADH, a reducing agent required in a variety of metabolic activities [16]. More, CA functions as a non-enzymatic antioxidant and facilitating the degradation of free radicals generated in plants under abiotic stress [17]. In this context, Zrig et al. [18] have showed that CA may improve salt tolerance in different plants by enhancing antioxidant enzyme activities. In another study, it is suggested that CA, as an antioxidant, enhanced leaf growth, and phosphorous, nitrogen and potassium contents in palm tree [19].
Consequently, the aim of this study was to find cost-effective remedies to reduce salt harmful effects by citric acid application. Further, studies using citric acid as promoter of growth are needed to describe how CA mediates the recovery or enhancement of resistance ability of plants to salinity. It is crucial too, to verify CA effects on the physio-biochemical plant characteristics and the activity of enzymes associated with oxidative stress under salinity. Physio-chemical characteristics in Moringa oleifera, is of utmost importance because it is directly related to plant medicinal properties.
2 Materials and methods
2.1 Plant material and growth conditions
The experiment was carried out at the research area of National Institute of Research in Rural Engineering, Waters and Forests (INRGREF) of Tunis (Tunisia). Imbibed seeds in distilled water for 48h were disinfected and placed in petri dishes to germinate. Collected homogenate germinated seeds were cultivated in pots placed in greenhouse at 22 °C with 16h photoperiod and 65% relative humidity. During the first two weeks, Hoagland solution was used for irrigation. Then, the seedlings were divided into three groups: The first group was irrigated with a control solution (Hoagland solution), the second with a salt solution (10 g/l NaCl) and the third group with a mixed solution (10 g/l NaCl and 100 mM CA.
2.2 Biochemical measurements
2.2.1 Photosynthetic pigments, soluble proteins, free amino acids and citric acid contents determination
The total chlorophyll content was determined using the Arnon method [20]. The absorbance of each sample was measured at 645 and 663 nm using spectrophotometer (PG instrument, T60 UV-V/United Kingdom).
The soluble proteins content was determined according to Bradford method [21]. Fresh samples were extracted in mixture of Tris–HCL buffer (50mM, pH 7.5), PVP and β-mercaptoethanol. The principe is based on employing coomassie brilliant blue in conjunction with bovine serum albumin, which served as the protein standard. Absorbance is measured at 595nm.
The quantification of free amino acids was conducted based on Ninhydrin method explained by Moore and Stein [22]. The supernatant of the extracted fresh mass in ethanol (80%) was used to dose the free amino acids. The optical density was measured at 520nm.
The estimation of citric acid concentrations was conducted in accordance with the methodology proposed by Yan-Lin and Soon-Kwan [23]. Mixture of methanol,chloroform and water (12/5/1, v/v/v, MCW) was used to extract citric acid. Measurement of absorbance was done at 420 nm.
2.2.2 Soluble sugar, malondialdehyde (MDA), hydrogen peroxide and proline contents determination
The quantification of total soluble sugar content was conducted in accordance with the methodology established by Yemm and Willis [24]. Total soluble sugars are measured using the phenol method in the presence of anthrone and sulfuric acid. Absorbance was measured at 640nm.
The quantification of MDA content was conducted using the measurement of ThioBarbituric Acid-Reacting Substances (TBARS), following the methodology outlined by Heath and Packer [25]. MDA was extracted with Trichloroacetic acid. Spectrophotometric measures were realised at 532 nm and 600 nm (non-specific value at 600 nm).
The quantification of hydrogen peroxide (H2O2) concentration was carried out using the methods published by Patterson et al. [26]. Colorimetric measure was realised at 508 nm based on H2O2 as a standard.
The determination of proline content was conducted using the methodology proposed by Bates et al. [27]. Proline extraction from dry matter samples was realised with sulphosalycylic acid. A spectrophotometric measure at 520 nm is done based on the complexation of proline with Ninhydrin.
2.3 Superoxide dismutase and catalase activities assays
The activity of superoxide dismutase (SOD; EC: 1.15.1.1) was assessed by measuring its ability to block the photochemical reduction of fi-nitroblue-tetrazolium chloride (NBT), using the technique described by Beyer and Fridovich [28].
The activity assay of catalase (CAT; EC: 1.11.1.6) was conducted in accordance with the methodology established by Cakmak [29]. Activity was measured in a reaction solution containing mixture of H2O2 and K2HPO4 buffer.
2.4 Statistical analysis
The data depicted in the figures are the average of five replicates per treatment and means ± confidence limits at alpha = 0.05 level. To compare and determine significant differences between means, at probability level ≤ 0.05, one-way analysis of variance (ANOVA) and Tukey’s HSD tests were used. SPSS software version 20.1(IBM version 20.0.2004) was used.
3 Results
3.1 Plant growth
NaCl treatment reduced plant growth. Where as, the supply of CA under salinity condition restored plant growth (Fig. 1). Control plant remains taller than co-treated plants.
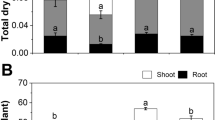
Salt reduced FW in shoot by more than 50%. The co-treatment salt-CA, alleviated the salt effect; shoot FW decreased by only 25% (Fig. 2A). Additionally, salt reduced severely total chlorophyll in shoots by more than 70% (Fig. 2B). The co-treatment salt-CA mitigated the salt effect. Under co-treatment, total chlorophyll decreased only by 42% referring to control.
Variation of fresh weight (A), total chlorophyll (B) and citric acid contents (C) in leaves of Moringa oleifera cultivated under different treatments (control, NaCl and co-treatment salt-citric acid). Data were subjected to ANOVA analysis and Tukey’s HSD test at 5% probability. Data are means of five replicates. SPSS software version 20.1(IBM version 20.0.2004) was used
Salinity reduced too CA content in shoots (45%). Under salt treatment, the supply of CA in irrigate water enhance CA accumulation in shoots referring to salt-treated shoot (Fig. 2C). The decrease of CA content in co-treated shoot was only 16% refer to control.
3.2 Soluble proteins, free amino acids, proline and soluble sugars contents
Plant soluble proteins that serve as enzymes and hormones are primarily vulnerable to stress conditions. Under salt conditions, total soluble protein decreased by 67% refereeing to control (Table 1). In co-treated shoots, a small decrease in total soluble protein was detected (2%).
Plants under stress accumulate more free amino acids, such as proline. Accumulated amino acids in plants serve numerous roles, primarily osmolytes. Free amino acid contents increased under salt treatment by 15% referring to control shoots. The free amino acid level decreased in co-treated shoots, but only by 10% referring to control (Table 1).
Soluble sugars are accumulated in plant tissues and cells due to salinity stress. They may act as an osmotic conservation factor.
Proline level and soluble sugar content increased under salt stress by more than the double referring to control. The co-treatment increased proline and soluble sugar contents, but the increase was much less than salinity. Proline and soluble sugar contents increased respectively by 85% and 60% (Table 1).
3.3 Malondialdehyde (MDA) and hydrogen peroxide (H2O2) contents
In living organisms, lipid oxidation produces, naturally, MDA. Under oxidative stress, lipid oxidation takes place and leading to fatty acid oxidation and MDA production. Under salt stress MDA level increased immensely to reach 67umol/g FW (Fig. 3A). The CA supply reduced the rise of MDA level (35 umol/g FW). The Fig. 3B illustrated the increase of H2O2 under salt stress: H2O2 level increased 3 times. Under the co-treatment, H2O2 level increase was much less than under only salt treatment (80% referring to control).
Malondialdehyde (MDA) (A) and hydrogen peroxide (H2O2) (B) contents in leaves of Moringa oleifera cultivated under different treatments (control, NaCl and co-treatment salt—citric acid). Data were subjected to ANOVA analysis and Tukey’s HSD test at 5% probability. Data are means of five replicates. SPSS software version 20.1(IBM version 20.0.2004) was used
3.4 Superoxide dismutase (SOD) and catalase (CAT) activities
SOD is a significant antioxidant enzyme that plays a crucial role in revealing harmful oxidative effects on plant cell. Its primary function involves catalyzing the dismutation of superoxide anions to produce hydrogen peroxide (H2O2) and dioxygen (O2).
Based on our data (Fig. 4A) SOD activity increased under salinity. The same enzyme activity increased 10 times refers to control (Fig. 4A). In fact, the SOD activity increase explains the showed H2O2 accumulation in salt treated shoots (Fig. 3B). The co-treatment CA-salt reduced the salt effect on SOD activity. Under co-treatment, SOD activity increased only 5 times refers to control. Compared top control conditions, CAT activity increased more than 5 times under salt treatment (Fig. 4B). Neverthelss, citric acid presence attenuated the negative effect of salt: CAT activity increased only 3 times.
Superoxide dismutase (SOD) (A) and catalase (B) activities in leaves of Moringa oleifera cultivated under different treatments (control, NaCl and co-treatment: salt—citric acid). Data were subjected to ANOVA analysis and Tukey’s HSD test at 5% probability. Data are means of five replicates. SPSS software version 20.1(IBM version 20.0.2004) was used
3.5 Means difference significance and correlation analysis
Data presented in (Table 2) demonstrates the one-way ANOVA of citric acid supply and salt stress condition, with measured parameters; shoot fresh weight, biochemical compounds contents and antioxidative enzymes activities. Results showed that the combination of adding citric acid and applying salt had a statistically significant effect at the 0.05 significance level.
To investigate the association between all measured parameters Pearson correlation analysis (Table 3) and correlations among variables (Table 4) were adopted. A significant correlation at the 0.01 level (2-tailed) was determined between the different culture conditions and the different measured variables. A positive correlation was noticed between the several variables measured in M. oleifera grown under the different conditions.
4 Discussion
Moringa oleifera L. widespred in tropical and subtropical regions. It is utilized all over the world as a multi-functional herbal plant for human sustenance and as an alternative medical plant. This tree’s leaves contain a variety of antioxidants, including ascorbic acid, carotenoids, phenolics, and flavonoids [30]. Additionally, according to its biochemical features, moringa leaf extract is used to improve plant growth and yield [31]. Razis et al. summarized the potential health Moringa benefits, particularly their antioxidant and anti-microbial properties. Because of its economic importance, multiple studies have concentrated on enhancing this plant cultivation under various constraints such as salt stress [32]. Salinity constitutes a significant abiotic plant stress, reducing crop productivity and alters plant physiology and morphology [33].
In current study, results showed that NaCl treatment reduced plant growth. Plant growth is influenced by salts via hormonal imbalances, ion toxicity, osmotic exertion, nutritional motivation reduction, and the formation of reactive oxygen species (ROS) [34]. Figure 2A showed that salinity reduced shoot fresh weight. Recent studies reported that salinity decreased various growth parameters of Moringa oleifeira: plant height, leaves number, stem diameter, and dry weight of leaves, stems and roots [35, 36]. The supply of CA, under salinity, improved pant growth (Fig. 1). Control plant remained taller than co-treated plants (CA + Salt). In several plant species, CA has been employed as a strategy to alleviate salt- adverse effects [37,38,39].
Currently, salt reduced FW in shoot by more than 50%. The co-treatment salt-CA alleviated the salt effect; shoot FW decreased by only 25% (Fig. 2A). Additionally, salt reduced severely total chlorophyll content (Fig. 2B). Recently, according to Bashir et al., salinity affected photosynthesis in Moringa by reducing chlorophyll a fluorescence and suppressing the electron transport pathway of PSII [40].
Under co-treatment, total chlorophyll level declined less compared to salt-treated seedlings. So, CA might mitigate harmful salt effects in Moringa plant. The use of citric acid as foliar spray or in irrigation water improved salt tolerance in several plant species [37, 39].
According to many experimental data, abiotic stress could affect endogenous level of citric acid and so generate oxidative stress [8]. In Fig. 2C, salinity reduced endogenous CA content in shoots (45%). The effect of salinity on endogenous CA levels varied across different plant species. Salinity exposure could increase endogenous CA levels in many plant species, including tomato and sunflower [41, 42]. In this case, citric acid as a small organic molecule could surely function as an osmolyte. In the current study, salinity decreased the CA rate, which explains the oxidative stress caused by salinity, leading to an increase in MDA content and a decrease in shoot growth.
Under salt treatment, the supply of CA enhanced endogenous CA accumulation in shoots referring to salt-treated shoot (Fig. 2C). The rise in CA level could explain the alleviation of salinity effect on both oxidative stress and growth.
In co-treated shoots, the decrease in total soluble protein was less than that detected in salt-treated shoots (Table 1). Current results were comparable to those found recently in wheat under salt stress and alleviated by ascorbin [43]. Free amino acid contents increased under salt treatment (Table 1). This rise in amino acid level was linked to a decrease in soluble proteins concentration reported under salinity. In fact, salt stress stimulates protease activity, leading to a reduction in soluble protein and an increase in free amino acids due to protein degradation [44].
In co-treated shoots, a decrease in the level of free amino acids is associated with a restoration of soluble protein levels, indicating a decrease in protease activity (Table 1). This confirmed that CA supply also alleviate adverse effect of salt on soluble protein and amino acid levels [43, 45].
Proline level and soluble sugar content increased under salt stress by more than the double referring to control. As well, Azeem et al. found that salt increased, proline and soluble sugar levels in Moringa [13]. In current study, Proline and soluble sugars could function as osmolyte to decrease osmotic potential. In fact, plants released osmotic active synthesis (OSMOPROS) in response to salinity stress to decrease osmotic potential. OSMOPROS’ major purpose is to maintain cellular turgor, which acts as a driving force for water absorption and can also act as a free radical.
The salt-treated shoots exhibited a greater increase in proline and soluble sugar contents than the co-treated shoots. This result suggests that co-treatment of plants with CA mitigated osmotic stress.
MDA, a byproduct of lipid oxidation, is typically produced in response to oxidative stress. Under salt stress, MDA level increased immensely to reach 67umol/g FW (Fig. 3A). The increase of MDA level under salinity was reported in Malus species [45]. Figure 3B depicted a significant rise in H2O2 concentration in response to salt stress. Multiple studies have documented that salt treatment induced oxidative damage in plants, as evidenced by elevated levels of MDA and H2O2 [13, 45].
In co-treated shoots, the reduction in MDA level is directly linked to the drop in H2O2 level with refer to salt-treated shoots. In saline conditions, the MDA level decrease by CA supply was reported in date palm tree [39]. This result suggested that CA supply mitigated oxidative stress generated under salinity [46].
SOD enzyme catalyzes superoxide dismutation and produce H2O2 and O2 [40]. SOD activity and CAT activity increased under salinity (Fig. 4A). The SOD activity increase explained the H2O2 accumulation in salt treated shoots (Fig. 3B). In Malus species too, salt treatment increased SOD activity [45]. The co-treatment CA-salt reduced the salt effect on SOD and CAT activities. When comparing co-treatment to salt treatment, the rise in SOD and CAT activities was lower in co-treated shoots. Indeed, antioxidant activity increased by salt, could be attenuated by several treatments including CA in current study, amino acid foliar spray [36]; salicylic acid [47], antioxidants [39], iron foliar spray [48] and nitrogen avialability [49]. CA modulated salt effect in several species including date palm [39], cotton [38], papaya [50] common bean [51], Mays [52], and Chinese ryegrass [37].
5 Conclusion
Exogenous application of citric acid has the ability to attenuate the negative effects of salt stress on Moringa oleifera. The mitigating effect of citric acid is shown by growth increase and a decrease in osmoregulator accumulation. In fact, diminution of proline and soluble sugar amounts when citric acid was added, reflected, that the cellular homeostasis state was more equilibrate than in salt-treated plants. Also, the decrease in MDA and H2O2 levels indicated that CA supply could protect cell membranes from salt damage by reducing lipid peroxidation. CA supply resulted too, in a reduction of antioxidant enzyme activities (SOD and CAT), indicating a reduced oxidative state. The supply of citric acid could be one of the less-cost solutions to mitigate the harmful effects of salt stress in Moringa.
Data availability
Our manuscript has no associated data.
References
Mukhopadhyay R, Sarkar B, Jat HS, Sharma PC, Bolan NS (2021) Soil salinity under climate change: challenges for sustainable agriculture and food security. J Environ Manage 280:111736. https://doi.org/10.1016/j.jenvman.2020.111736
Mbarki S, Skalicky M, Vachova P, Hajihashemi S, Jouini L, Zivcak M, Tlustos P, Brestic M, Hejnak V, Zoghlami KA (2020) Comparing salt tolerance at seedling and germination stages in local populations of medicago ciliaris L. To Medicago intertexta L. and Medicago scutellata L. Plants 9:526–534. https://doi.org/10.3390/plants9040526
Abou-Shlell MK, El Emary FA, Khalifa AA (2020) Effect of nanoparticle on growth, biochemical and anatomical chracteristics of Moringa plant (Moringa Oleifera) under salinity stress conditions. Archi agrice scien j 220:186–213. https://doi.org/10.21608/aasj.47687.1044
Hao S, Wang Y, Yan Y, Liu Y, Wang J, Chen S (2021) A review on plant responses to salt stress and their mechanisms of salt resistance. Hortic 7(6):132. https://doi.org/10.3390/horticulturae7060132
Chourasia KN, More SJ, Kumar A, Kumar D, Singh B, Bhardwaj V, Kumar A, Kumar-Das S, Singh RK, Zinta G, Tiwari RK, Lal MK (2022) Salinity responses and tolerance mechanisms in underground vegetable crops: an integrative review. Planta 255:68. https://doi.org/10.1007/s00425-022-03845-y
Mangal V, Lal MK, Tiwari RK, Altaf MA, Sood S, Kumar D, Aftab T (2023) Molecular insights into the role of reactive oxygen, nitrogen and sulphur species in conferring salinity stress tolerance in plants. J Plant Grow Reg 42:554–574. https://doi.org/10.1007/s00344-022-10591-8
Fahad S, Bano A (2012) Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pak J Bot 44:1433–1438
Ul-Arif TM, Zahan MI, Karim MM, Imran S, Hunter CT, Islam MS, Mima Hannan MA, Rhaman MS, Hossain MA (2021) Citric acid-mediated abiotic stress tolerance in plants. Int J Mol Sci 22:7235. https://doi.org/10.3390/ijms22137235
Gohari G, Panahirad S, Sepehri N, Akbari A, Zahedi S-M, Jafari H, Fotopoulos V (2021) Enhanced tolerance to salinity stress in grapevine plants through application of carbon quantum dots functionalized by proline. Envir Sci Pollu Res 28:42877–42890. https://doi.org/10.1007/s11356-021-13794-w
Liu C, Mao B, Yuan D, Chu C, Duan M (2022) Salt tolerance in rice: Physiological responses and molecular mechanisms. The Crop J 10:13–25. https://doi.org/10.1016/j.cj.2021.02.010
Fahad S, Danish S, Datta R, Saud S, Lichtfouse E (2023) Biochar to improve crop production and decrease plant stress under a changing climate. Springer Nature Switzerland AG. https://doi.org/10.1007/978-3-031-26983-7
Padayachee B, Baijnath HJSAJOB (2020) An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. South Afr J Bot 129:304–316
Azeem M, Pirjan K, Qasim M, Mahmood A, Javed T, Muhammad H, Rahimi M (2023) Salinity stress improves antioxidant potential by modulating physio-biochemical responses in Moringa oleifera Lam. Sci Rep 13(1):2895. https://doi.org/10.1038/s41598-023-29954-6
Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, Khan FA, Khan F, Chen CY, Wu TMA, Chun MX, Afzal M, Jan A, Jan MT, Huang J (2015) Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ Sci Pollut Res Int 22(7):4907–4921. https://doi.org/10.1007/s11356-014-3754-2
Faheed FA, Hassanein AM, El-nagish AA, Salem J (2020) Salicylic acid-mediated salt stress tolerance by mitigation of the oxidative effects in Moringa. J Env Stud 20(1):7–20
Zhong M, Yuan Y, Shu S, Sun J, Guo S, Yuan R, Tang Y (2016) Effects of exogenous putrescine on glycolysis and Krebs cycle metabolism in cucumber leaves subjected to salt stress. Plant Growth Regul 79:319–330. https://doi.org/10.1007/s10725-015-0136-9
Faraz A, Faizan M, Sami F, Siddiqui H, Hayat S (2020) Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. J Plant Grow Reg 39:641–655. https://doi.org/10.1007/s00344-019-10007-0
Taaima M-H (2021) The effect of treatment with citric acid at different levels on growth indicators and leaf content of mineral elements of date palm cuttings (Phoenix Dactylifera L.) Al-Sayer variety. Annals Rom Soc for Cell Bio 4377–4388. http://www.annalsofrscb.ro/index.php/journal/article/view/1459.
Zrig A, AbdElgawad H, Touneckti T, Mohamed H-B, Hamouda F, Khemira H (2021) Potassium and calcium improve salt tolerance of Thymus vulgaris by activating the antioxidant systems. Sci Hortic 277:109812. https://doi.org/10.1016/j.scienta.2020.109812
Arnon D-I (1949) Copper enzyme in isolated chloroplasts: Polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC437905/
Bradford M-M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Bioch 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Moore S, Stein W-H (1954) Procedure for the chromatographic determination of amino acids on four per cent. Crosslinked sulfonated polystyrene resins. Journal of Biological Chemistry 25(3). 211: 893–906 https://www.cabdirect.org/cabdirect/abstract/19551403201
Yan-Lin S, Soon-Kwan H (2011) Effects of citric acid as an important component of the responses to saline and alkaline stress in the halophyte Leymus chinensis (Trin.). Plant Growth Regul 64:129–139
Yemm E-W, Willis A-J (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514. https://doi.org/10.1042/bj0570508
Heath R-L, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Patterson B-D, MacRae E-A, Ferguson I-B (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Analy biochem 139:487–492. https://doi.org/10.1016/0003-2697(84)90039-3
Bates L-S, Waldren R-A, Teare I-D (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Beyer J-W-F, Fridovich I (1987) Effect of hydrogen peroxide on the iron-containing superoxide dismutase of Escherichia coli. Biochem 26:1251–1257. https://doi.org/10.1021/bi00379a008
Cakmak I, Strabac D, Marccner H (1993) Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J Exp Bot 44:127–132. https://doi.org/10.1093/jxb/44.1.127
Vongsak B, Sithisam P, Gritsanapan W (2014) Simultaneous HPLC quantitative analysis of active compounds in leaves of Moringa oleifera Lam. J Chromatogr Sci 52:641–645. https://doi.org/10.1093/chromsci/bmt093
Mehmood A, Naveed K, Ayub Q, Alamri S, Siddiqui MS, Wu C, Wang D, Saud S, Banout J, Danish S, Datta HHM, Nasim W, Mubeen M, Shah F, Fahad S (2021) Exploring the potential of Moringa leaf extract as bio stimulant for improving yield and quality of black cumin oil. Sci Rep 11:24217. https://doi.org/10.1038/s41598-021-03617-w
Razis AA, Ibrahim MD, Kntayya SB (2014) Health benefits of Moringa oleifera. Asian Pac J Cancer Prev 15:8571–8576. https://doi.org/10.7314/APJCP.2014.15.20.8571
El-Serafy RS, El-Sheshtawy AA, Atteya AK, Al-Hashimi A, Abbasi AM, Al-Ashkar I (2021) Seed priming with silicon as a potential to increase salt stress tolerance in Lathyrus odoratus. Plants 10:2140. https://doi.org/10.3390/plants10102140
Kumar A, Singh S, Gaurav A-K, Srivastava S, Verma J-P (2020) Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front Microbiol 11:1216. https://doi.org/10.3389/fmicb.01216
Atteya AKG, El-Serafy RS, El-Zabalawy KM, Elhakem A, Genaidy EAE (2022) Exogenously supplemented proline and phenylalanine improve growth, productivity, and oil composition of salted Moringa by up-regulating osmoprotectants and stimulating antioxidant machinery. Plants 11:1553
Abou-Shlell MK, El-Emary FA, Khalifa AA (2020) Effect of nanoparticle on growth, biochemical and anatomical characteristics of Moringa plant (Moringa oleifera L.) under salinity stress condition. Arch Agric Sci J 3:186–213. https://doi.org/10.21608/aasj.2020.47687.1044
Sun YL, Hong SK (2011) Effects of citric acid as an important component of the responses to saline and alkaline stress in the halophyte Leymus Chinensis (Trin.). Plant Growth Regul 64:129–139. https://doi.org/10.1007/s10725-010-9547-9
El-Beltagi HS, Ahmed SH, Namich AAM, Abdel-Sattar RR (2017) Effect of salicylic acid and potassium citrate on cotton plant under salt stress. Fresen Environ Bull 26: 1091–1100. https://scholar.cu.edu.eg/sites/default/files/hossam-el-beltagi/files/67feb_16_635_1
Shareef HJ, Muayed F, Abbas M (2022) Response of date palm offshoots (Phoenix dactylifera L.) to the foliar spray of salicylic acid and citric acid under salinity conditions. Fol Oec. https://doi.org/10.2478/foecol-2022-0015
Bashir S, ulMisbah A, Faiza B, Muhammad J, Adnan H, Saba F, Rabia P, Aneela KS, Shehrooz A, Shumaila R, Tooba H, Ayesha I, Ayesha P, Atiq-ur-Rehman A A, Zafar UZ, Habib RA (2021) Structural and functional stability of photosystem-II in Moringa oleifera under salt stress. AJCS 15:676–682 ISSN:1835–2707 https://doi.org/10.21475/ajcs.21.15.05. p 2996
Shi D, Sheng Y (2005) Effect of various salt-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ Exp Bot 54:8–21. https://doi.org/10.1016/j.envexpbot.2004.05.003
Mickky BM, Abbas MA, Sameh NM (2019) Morpho-Physiological status of fenugreek seedlings under NaCl stress. J King Saud Univ Sci 31:1276–1282. https://doi.org/10.1016/j.jksus.2019.02.005
Dadrwa BK, Bagdi DL, Kakralya BL, Sharma MK (2022) Foliar treatment with ascobin reduces the adverse effects of salt stress on physiological and biochemical parameters in wheat. The Pharma Innovn J 11(5): 2117–2120. https://www.thepharmajournal.com/archives/2022/vol11issue5/PartX/1 5:231–519.
Mozanzadeh MT, Safari O, Oosooli R, Mehrjooyan S, Najafabadi MZ, Hoseini SJ, Monem J (2021) The effect of salinity on growth performance, digestive and antioxidant enzymes, humoral immunity and stress indices in two euryhaline fish species: Yellowfin seabream (Acanthopagrus latus) and Asian seabass (Lates calcarifer). Aquaculture 534:736–329
Wang WX, Zhang ZX, Wang X, Han C, Dong YJ, Wang YX (2023) Functional identification of ANR genes in apple (Malus halliana) that reduce saline–alkali stress tolerance. Plant Biol 25:892–901. https://doi.org/10.1111/plb.13559
Li SJ, Wang WL, Ma YC, Liu SC, Grierson D, Yin XR, Chen KS (2020) Citrus CitERF6 contributes to citric acid degradation via upregulation of CitAclα1, encoding ATP-citrate lyase subunit α. J Agric Food Che 68:10081–10087. https://doi.org/10.1021/acs.jafc.0c03669
Ahmad F, Kamal A, Singh A, Ashfaque F, Alamri S, Siddiqui MH (2020) Salicylic acid modulates antioxidant system, defense metabolites, and expression of salt transporter genes in Pisum sativum under salinity stress. J Plant Gro Reg. https://doi.org/10.1007/s00344-020-10271-5
Tawfik MM, Mohamed MH, Sadak MS, Thalooth AT (2021) Iron oxide nanoparticles effect on growth, physiological traits and nutritional contents of Moringa oleifera grown in saline environment. Bull Nat Res Centre 45:1–9. https://doi.org/10.1186/s42269-021-00624-9
Hajaji AN, Bouthour D, Hfaidh R, Gouia H, Pageau K, Haouari CC (2013) The role of nitrogen availability for the salt-tolerance of two different varieties of Durum Wheat. Bull Environ Contam Toxicol 91:711–717. https://doi.org/10.1007/s00128-013-1120-
Zanotti RF, Lopes JC, Motta LB, de Freitas AR, Mengarda LHG (2013) Tolerance induction to saline stress in papaya seeds treated with potassium nitrate and sildenafil citrate. Semina: Ciências Agrárias 1: 3669–3673. https://doi.org/10.5433/1679-0359.2013v34n6Supl1p3669
El-Tohamy WA, El-Abagy HM, Badr MA, Gruda N (2013) Drought tolerance and water status of bean plants (Phaseolus vulgaris L.) as affected by citric acid application. J Appl Bot Food Qual 86:10–5073. https://doi.org/10.5073/JABFQ.2013.086.029.
El-Hawary MM, Nashed ME (2019) Effect of foliar application by some antioxidants on growth and productivity of maize under saline soil conditions. J Plant Prod 10:93–99. https://doi.org/10.21608/jpp.2019.36238
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Nasraoui Hajaji Afef and Ammari Youssef contributed to the study conception and design. Experiment realization, data collection and analysis, were performed by Nasraoui Hajaji Afef. Maaroufi Dguimi Houda wrote the first draft of the manuscript. Nasraoui Hajaji Afef revised manuscript and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hajaji, A.N., Maaroufi-Dguimi, H. & Ammari, Y. Exogenous application of citric acid mitigates salt-induced oxidative stress in Moringa oleifera seedlings. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00169-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-024-00169-3