Abstract
Since many cultivated plants, including rice, are susceptible to stress and salt stress, resulting in a tremendous reduction in yield, threatens food security worldwide. Strategies such as using biostimulants to ameliorate salt stress can reduce stress effects and sustain production. The effects of soaking Koshihikari (salt-sensitive) seeds in astaxanthin (AS) under salt stress were determined in the present study. In particular, the seeds of the rice cultivar were subjected to control, salt stress (50 mM NaCl), AS (50 µM), and AS + salt stress treatments for two weeks in hydroponic culture. Thereafter, the plants were harvested, and their growth, physiological, and molecular parameters were analyzed. The results showed that the growth of plants under salt stress was significantly reduced; however, the growth was restored to levels comparable to those of non-stressed plants treated with AS. Salt stress significantly increased the concentrations of malondialdehyde, hydrogen peroxide, and the electrolyte leakage ratio in untreated plants and significantly decreased their concentration in AS-treated plants under the same conditions, with corresponding increases in leaf catalase, peroxidase, and ascorbate peroxidase activities. Leaf Na+ concentration markedly increased under salt stress in non-treated plants, and AS treatment reduced the concentration. However, the difference was not statistically significant, which resulted in a significant decrease in the Na+/K+ ratio in AS-treated plants compared to that in non-treated plants. Salt stress and AS treatment did not alter the concentration of photosynthetic pigments but enhanced the expression of OsBHY, OsNHX1, OsSOS1, and OsHKT1;5 genes. Overall, soaking seeds in AS induced salt stress tolerance in the Koshihikari rice cultivar by reducing oxidative stress damage and enhancing shoot Na+/K+ balance. Therefore, seed-soaking methods using AS could serve as a good strategy for improving the cultivation of salt-sensitive rice cultivars in saline soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ensuring global food security is increasingly challenging as large areas of arable lands worldwide are facing ever-increasing environmental stress conditions, such as water deficit, salinization, and alkalization (Fang et al. 2021). In addition to these constraints, climate change has markedly contributed to lower yields of many agricultural products. Therefore, new management practices that will boost crop production under these adverse conditions are important to meet the demands of the ever-increasing world population, which is expected to reach 9–11 billion people by 2050 (Röös et al. 2017). Salinity is among the major contributors to the decline in crop production worldwide. It has been the subject of immense scientific research to generate tolerant varieties by introducing salt stress-tolerant genes for the sustainable production of crops under stress conditions (Sriskantharajah et al. 2020). Such cultivars are highly needed as salinity encroaches on arable land. However, most crops are glycophytes, requiring halophytic trait transfer for growth and reproduction under these conditions (Himabindu et al. 2016; Mangu et al. 2019).
Glycophytes are susceptible to salt stress (Nampei et al. 2021). Salt stress affects plants through (1) excess and uncontrolled Na+ transport from the root to photosynthetic tissues, where it induces diverse metabolic damages, including chlorophyll biosynthesis and enzyme deactivation, and compromises membrane integrity (Munns and Tester 2008; Yang et al. 2019); (2) induction of osmotic stress owing to lowered soil osmotic potential due to high concentrations of salts, which inhibit water uptake by roots and consequently decrease net photosynthetic output leading to growth reduction (Munns and Tester 2008); (3) induction of oxidative stress due to free radicals (especially superoxide anions (O2−) that accumulate along electron transport pathways in chloroplasts (affecting photosynthesis), mitochondria (affecting respiration), and peroxisomes (affecting photorespiration), thereby orchestrating damage to cell membrane lipids, DNA, and proteins, which sometimes end in cell death (Gill and Tuteja 2010); and (4) mineral deficiency owing to inhibited water uptake (Shams et al. 2019).
Tolerant glycophytes and halophytes have evolved several mechanisms to overcome these salt-induced challenges. These include shoot Na+ exclusion (restricted transport of Na+ to shoot from roots) mediated by high-affinity K+ transporters (HKT) and the salt overly sensitive 1 (SOS1) antiporter (Assaha et al. 2017a; Munns et al. 2020a); tissue tolerance (Na+ uptake in the shoot and its vacuolar sequestration through tonoplast Na+/H+ exchangers such as NHX1) (Assaha et al. 2017b); reactive oxygen species (ROS) scavenging through the removal of free oxygen radicals and derivatives, such as O2−, H2O2, and OH• by enzymes (catalase, CAT; ascorbate peroxidase, APX; superoxide dismutase, SOD; and peroxidase, POD) and non-enzymes (e.g., proline, glycine betaine, and carotenoid phenolics) (Gill and Tuteja 2010); salt secretion via salt glands (Shabala et al. 2014); Na+ recirculation from the plant to the soil (Fujimaki et al. 2015); and accumulation of organic and inorganic solutes to counter external osmotic potential to enable sustained water and mineral uptake (Munns et al. 2020b). However, as mentioned earlier, most crops are glycophytes and would suffer under high salinity conditions because they lack one or more of these tolerance traits (Chuamnakthong et al. 2019).
Rice (Oryza sativa L.) is a saline susceptible plant (Horie et al. 2012). It is a staple crop in many parts of the world; however, its production is declining because of its susceptibility to salt stress (Kobayashi et al. 2018). Accordingly, there has been a preponderance of research on improving the stress tolerance of this crop. The response to salinity varies among the hundreds of rice cultivars that have been generated to date, with some being more tolerant than others, especially wild varieties (Prusty et al. 2018; Tahjib-Ul-Arif et al. 2018). Hence, stress tolerance genes have been successfully engineered to enhance tolerance in susceptible cultivars (Munns et al. 2012). However, due to restrictions on using genetically modified organisms (GMOs) in many countries, this practice has yet to gain global acceptance (Toft 2012); therefore, the continued search for other stress tolerance strategies remains important. In addition, stress alleviation strategies to boost the production of salt stress-susceptible rice cultivars are needed. Koshihikari is a rice cultivar susceptible to salt stress; however, it is one of the most prized cultivars in many countries, including Japan, Australia, and America (Kobayashi et al. 2018). This cultivar is sensitive because salt stress induces reduced root Na+ efflux, increased K+ efflux, and physical damage to root tissues, leading to a reduced ability to sequester Na+ at the root and regulate the amount reaching the shoot from the root, resulting in an increase in shoot Na+ accumulation (Liu et al. 2019). In the absence of Na+ sequestration, osmotic adjustment, and an antioxidant defense system, this uncontrolled Na+ uptake and translocation to the shoot leads to reduced biomass and yield of the cultivar (Akter and Oue 2018; Mekawy et al. 2018; Mitsuya et al. 2019). It also leads to low K+/Na+ ratios owing to the reduced ability to retain K+ in the root (Liu et al. 2019), thereby exposing plants to the damaging effects of Na+ on K+-related functions. Therefore, optimizing Koshihikari production in saline environments requires treatments that alleviate these negative salt-induced traits.
Astaxanthin (AS; 3,3’-dihydroxy-4,4’-dione-β, β’-carotene) is a ketocarotenoid, synthesized from carotene through 3-ketolation and 4-hydroxylation (Seabra and Pedrosa 2010). It is produced by certain microalgae and yeasts and has very powerful antioxidant characteristics in both humans and animals. Therefore, AS is a potential anticancer agent used to enhance the nutritional quality of foods (Özbeyli et al. 2020). Oxidative damage is one of the major effects of salt stress on the growth and development of plants, and AS has been implicated in plant growth regulation and the mitigation of oxidative damage induced by salinity stress in strawberry tissue-cultured seedlings (Zhong et al., 2018). Moreover, the antioxidant capacity of AS has been demonstrated in transgenic rice plants (Zhu et al. 2018). Because of these beneficial effects, we hypothesized that using AS might impart salt stress tolerance to the salt-sensitive rice cultivar Koshihikari, especially because it has a deficient antioxidant defense barrier. Therefore, this study aimed to explore the ameliorative effects of AS on Koshihikari through seed soaking.
Materials and methods
Vegetal material and culture conditions
The salt-sensitive Koshihikari rice cultivar used in this study was obtained from a stock at the Plant Nutritional Physiology Laboratory of Hiroshima University. The seeds of the rice cultivar were surface-sterilized as previously described (Mekawy et al. 2018). The seeds were then split into two groups: Group 1 was immersed in different concentrations (5, 10, 50, and 100 µM) of AS (1 g/mL dimethyl sulfoxide, DMSO) in distilled water for 24 h at 28 \(^\circ\)C, whereas Group 2 was soaked in similar amount of DMSO and distilled water (unprimed). The seeds were then air-dried for 3 h and soaked in distilled water for 2 days. After germination, seeds 200 seeds of the rice cultivar were placed on plastic nets floating in half-strength Kimura B hydroponic nutrient solution (0.18 mM (NH4)2SO4, 0.27 mM MgSO4·7H2O, 0.09 mM KNO3, 0.18 mM Ca(NO3)2·4H2O, 0.09 mM KH2PO4, 20 µM NaEDTA-Fe·3H2O, 6.7 µM MnCl2·4H2O, 9.4 µM H3BO3, 0.015 µM (NH4)6Mo7O24·4H2O, 0.15 µM ZnSO4·7H2O, 0.16 µM CuSO4·5H2O) as previously reported (Mekawy et al. 2018). It was supplemented with 50 mM NaCl for the stress treatment. Unsupplemented Kimura B was used as the control (untreated). Nutrient solutions were renewed daily. The salt treatment was performed immediately after germination. Seeds soaked in different concentrations of AS were tested, for optimization purposes and a concentration of 50 µM was selected for the study. The experiment comprised four treatments [control (no AS), AS pretreatment, NaCl, and AS + NaCl] with 40 plants per treatment and four replicates. The pH of the nutrient solution was kept within the range of 5.0–5.5, and the plants were monitored for two weeks under the following conditions in a growth chamber: 70% relative humidity, 24 ± 2 °C temperature, and a photoperiod of 16 h light at a photosynthetic photon flux density of 250–350 µmol/m2/s and 8 h of darkness (Mekawy et al. 2018). Finally, the plants were harvested at the end of the two-week treatment period. Their growth (1 plant per replicate) and various physiological parameters (tissues from 2 plants per replicate) were measured in one plant per replicate per treatment.
Growth measurement
The lengths (cm) of the roots and shoots of plants under different treatments were measured using a graduated ruler. Thereafter, the plants were partitioned into roots, leaf sheaths, and leaf blades, and their fresh weights (FW) were measured. The samples were then oven-dried at 70 ℃ for 72 h and the dry weight (DW) was recorded. The dried samples were used for Na+ and K+ measurements. Other plant samples were flash-frozen in liquid nitrogen for storage at − 80 ℃ until usage in various physiological analyses.
Determination of sodium (Na+) and potassium (K+) concentrations
Na+ and K+ concentrations were measured in the leaf blades, leaf sheaths, and roots as previously described (Mekawy et al. 2015). The dried samples (~ 1 g) were gently agitated in 1 N HCl overnight and the Na+ and K+ ion contents were measured using a flame photometer (ANA-135; Tokyo Photoelectric, Tokyo, Japan). The concentrations of Na+ and K+ in the samples were calculated from the curves obtained from standard solutions of Na+ and K+.
Measurement of chlorophyll and carotenoid contents
Chlorophyll and carotenoids were extracted and measured as previously described (Assaha et al. 2017a; Mekawy et al. 2018). The second leaves from the tops were used for analysis. Extraction was performed in N,N-dimethyl formamide (DMF) using 500 mg of fresh leaf samples. The absorbance of the extracts was measured at 646.8, 663.8, and 480 nm. The concentrations were calculated using previously described methods (Porra et al. 1989; Wellburn 1994), as follows:
where Chl a = chlorophyll a, Chl b = chlorophyll b, Chl a + b = total chlorophyll, Car = carotenoids, and A = absorbance.
Determination of the electrolyte leakage ratio (ELR), hydrogen peroxide (H2O2) and malondialdehyde (MDA) concentrations
The ELR of fresh leaf samples from plants was determined according to previous reports (Mekawy et al. 2015). Briefly, the electrical conductivity (EC) of deionized water containing leaf segments was measured before (EC1) and after (EC2) autoclaving. Then the two EC values were used to calculate the ELR using Eq. 5.
H2O2 extraction and measurements were performed as previously described (Mekawy et al. 2018). The extraction was performed using ground leaf and root tissues in 4 mL of cold acetone. The resulting homogenate was mixed with a reaction buffer (0.25 mM FeSO4, 0.25 mM (NH4)2SO4, 25 mM H2SO4, 125 µM xylenol orange, and 10 mM sorbitol). Finally, H2O2 levels were read spectrophotometrically at 560 nm, and the concentration was calculated using reference standards (Suharsono et al. 2002). MDA concentrations were determined using the thiobarbituric acid (TBA) reaction as previously described (Assaha et al. 2015b; Mekawy et al. 2018). Fresh leaf and root samples (100 mg) were homogenized in an extraction buffer (10 mM HEPES, pH 7.0, 15% tricarboxylic acid, 0.375% TBA, 0.25 N HCl, 0.04% butylated hydroxyl toluene, and 2% ethanol), incubated at 95 °C and then centrifuged. The absorbance of the supernatant was read at 535 and 600 nm for nonspecific absorption, and the MDA content was calculated using the extinction coefficient (155 mM−1 cm−1).
Measurement of proline, total phenolics (TP), and total flavonoid (TF) concentrations
The proline concentration was measured using a previously described method (Bates et al. 1973), with l-proline as the standard. The TP was extracted and measured according to previously described methods (Ainsworth and Gillespie 2007; Assaha et al. 2017a). Briefly, leaf and root extracts were reacted with gallic acid, Na2CO3, and the Folin-Ciocalteu reagent. After incubation for 2 h, the absorbance of the mixture was read at 765 nm, and the concentration of TP was measured in terms of gallic acid equivalents. TFs were extracted and measured as previously described (Memari-Tabrizi et al. 2021). Briefly, a 2-mL mixture of leaf and root extracts and ethanol was combined with 3 mL of a reaction mixture composed of 10% AlCl3 and 1 M potassium acetate. After incubating the mixture for 0.5 h at room temperature, the absorbance was read at 415 nm, and the TF concentration was expressed as quercetin equivalents.
Measurement of antioxidant enzyme activities
Enzyme extraction was conducted using 0.5 g of fresh leaf and root tissue, according to previously reported methods (Assaha et al. 2015b; Mekawy et al. 2018; Takagi and Yamada 2013) to determine the activities of catalase (CAT; EC 1.11.1.6) and ascorbate peroxidase (APX; EC 1.11.1.11). The assay mixture for CAT consisted of the enzyme extract, H2O2, and potassium phosphate buffer (50 mM, pH 7.0). The activity was recorded as a decrease in H2O2 at 240 and expressed as mmol H2O2 consumed per minute. The oxidation of ascorbate at 290 nm was measured in a 1 mL reaction mixture consisting of phosphate buffer (25 mM, pH 7.0), ascorbic acid, EDTA, H2O2, and 10% enzyme extract to measure APX activity. the activity was calculated using 2.8 mM−1 cm−1 as the extinction coefficient and reported as µmol of ascorbate oxidized per minute. The increase in absorbance of 1 mL of the reaction mixture composed of guaiacol phosphate buffer, H2O2, and 2% enzyme extract was monitored at 470 nm for 1 min to measure POD activity. The activity was calculated using the extinction coefficient (26.6 mM−1 cm−1) for tetraguaiacol (Chance and Maehly 1955). The protein concentration in the enzyme extracts was measured using a protein assay kit (Bio-Rad DC, CA, USA), with bovine serum albumin as the standard, according to the manufacturer’s instructions.
Gene expression analysis
Gene expression analysis was performed as previously described (Assaha et al. 2015b; Mekawy et al. 2015, 2018). Leaf and root tissues of the control and stressed plants were ground to a powder using a mortar, pestle, and liquid nitrogen. Approximately 1 g of the powdered sample was used for total RNA extraction using a total RNA extraction kit (Plant) (RBC Bioscience, SciTrove, Japan) according to the manufacturer’s instructions. The extracted RNA was quantified using a Nanodrop spectrophotometer ND-1000 (Thermo Fisher Scientific, Inc.). Thereafter, 1 µg of the extracted RNA was used to synthesize first strand cDNA using the ReverTra Ace qPCR RT Kit (TOYOBO, Osaka, Japan). The synthesized cDNA was subsequently used in quantitative real-time polymerase chain reactions, using the Thunderbird SYBR qPCR mix (TOYOBO, Osaka, Japan), to quantify the expression levels of the Oryza sativa tonoplast Na+/H+ exchanger (OsNHX1) (Primer, F: 5′-TGGCTGCTGCTAATGAGTTG-3′, R: 5′-ACCAATCATCCCGAACCAT-3′), β-carotene hydroxylase (BHY) (Primer, F: 5′-GGGATTACGCTGTTCGGGAT-3′, R: 5′-TGTGATGTATCTGGTGGGCG-3′), OsHKT1;5 (Primer, F: 5′-CCCATCAACTACAGCGTCCT-3′, R: 5′-AGCTGTACCCCGTGCTGA-3′), and OsSOS1 (Primer, F: 5′-ATACTGAGTGGGGTTGTTATTGC-3′, R: 5′-AAAGGTAAATTTCAAAAGGTACATGG-3′). The expression levels were determined using the 2−ΔΔCT method (Livak and Schmittgen 2001), with the OsUBQ5 gene (Primer, F: 5′-ACCACTTCGACCGCCACTACT-3′, R: 5′-ACGCCTAAGCCTGCTGGTT-3′) used as an internal control. The quantitative RT-PCR was performed using the following conditions: an initial incubation at 95 ℃ for 1 min, followed by 40 cycles of denaturation at 95 ℃ for 15 s and extension at 60 ℃ for 60 s. Melt curve analysis was performed to verify the PCR products.
Statistical analysis
Analysis of variance (ANOVA) was performed on all data obtained in this study using the SPSS statistical package version 21 (IBM Corp. Amonk, NY, USA) after normality testing. Means ± SE (n = 3) were compared using the Tukey HSD test at α = 0.05.
Results
Effect of AS on plant growth under saline and non-saline conditions
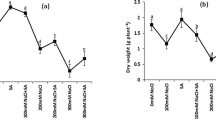
The effects of AS on the growth of rice plants grown under saline and non-stress conditions are shown in Fig. 1. The total dry mass of the plants was significantly reduced (38% reduction, p < 0.05) under salt stress, especially the mass of the roots and leaves (48 and 51% reduction, respectively). Although the application of 50 µM AS did not alter the dry weight of plants under non-saline conditions, it restored the DW of plants grown under salt stress to levels similar of that of the control (Fig. 1A). Salt stress also significantly reduced the total length of the plants (19% reduction, p < 0.05), whereas AS restored the length to levels comparable to those of control plants. However, under non-saline conditions, AS significantly increased the total and shoot lengths (13 and 17%, respectively) of rice plants compared with those of control plants (Fig. 1B).
Effects of AS on the growth of Koshihikari seedlings under saline and non-saline conditions. Effect of AS on A dry weight and B plant length. Bars represent means ± SE (n = 3), and bars with the same letter are not statistically different (p ≤ 0.05). C = control, S = salt stress, AS = astaxanthin, and ASS = astaxanthin + salt stress
Effect of AS on Na+ and K+ concentrations, and Na+/K+ ratio under saline and non-saline conditions
The concentrations of Na+ and K+ and the Na+/K+ ratio are presented in Fig. 2. Salt stress significantly elevated the concentration of Na+ in the leaves, sheaths, and roots of the plants (Fig. 2A, B, and C). However, the application of AS decreased the Na+ concentration in the leaves and sheaths by 30 and 31%, respectively, with no statistical significance, implying that AS may contribute to reduced shoot Na+ translocation. AS did not alter the concentration of Na+ in the roots under salt stress compared to that in the non-treated stressed plant roots. The Na+ concentration in shoots was higher than that in roots. Salt stress markedly decreased K+ concentration in the leaves, sheaths, and roots (1.5-, 3-, and 1.6-fold reductions, respectively) compared with the control (Fig. 2D, E, and F). However, AS application did not significantly alter K+ levels under either saline or non-saline conditions, implying that AS did not restore shoot K+ levels depleted due to salt stress. However, AS restored K+ concentrations in the roots to levels comparable to those in the controls. With reduced K+ concentrations in the different organs, salt stress significantly increased the Na+/K+ ratio in the leaves, sheaths, and roots compared to that in the organs of AS-treated plants, indicating that AS reduced the Na+/K+ ratio required for stress tolerance, especially in the shoots (Fig. 2G, H, and I).
Effect of AS on the concentration of Na+ (A, B, C) and K+ (D, E, F), and the Na+/K+ ratio (G, H, I) in the leaf, sheath, and root of rice plants grown under saline and non-saline conditions. Bars represent means ± SE (n = 3), and bars with the same letter are not statistically different (p ≤ 0.05). C = control, S = salt stress, AS = astaxanthin, and ASS = astaxanthin + salt stress
Effect of AS on chlorophyll (chl) and carotenoid concentrations under saline and non-saline conditions
The concentrations of Chl a, Chl b, total Chl, and carotenoids are shown in Fig. 3. Salt stress and AS did not significantly alter these concentrations compared to the control treatment, implying that the ameliorative effects of AS were unrelated to chlorophyll concentration.
Effect of AS on the concentration of Chl a, Chl b, and Total Chl (Chl a + b), and total carotenoids in the leaves of Koshihikari plants grown under saline and non-saline conditions. Bars represent means ± SE (n = 3) and bars with the same letter are not statistically different (p ≤ 0.05). C = control, S = salt stress, AS = astaxanthin, and ASS = astaxanthin + salt stress
Effect of AS on ELR, and H2O2 and MDA concentrations under saline and non-saline conditions
The effects of AS application on ELR, H2O2, and MDA production under non-saline and saline conditions are presented in Fig. 4. Salt stress significantly elevated the H2O2 concentration in the leaves of the from control by 220% and that of AS-treated plants by 59%. Thus, AS application reduced salt-induced H2O2 concentration by 29% (Fig. 4A), indicating that AS can lower salt stress-induced H2O2 production. H2O2 levels were markedly lower in the roots than in the leaves; however, this level increased under all treatments, especially in salt stressed AS-treated plants (47% increase), compared with to that in the control (Fig. 4B). The concentration of MDA in the leaves increased by 60% under salt stress and 40% in salt-stressed plants treated with AS (Fig. 4C), indicating that AS could abate salt-induced MDA production. The MDA concentration in the roots was not significantly altered by any of the treatments (Fig. 4D). Compared to the control, salt stress significantly increased the ELR of untreated plants by 15.7-fold and that of treated plants by 2.6-fold (Fig. 4E), suggesting that AS is involved in the protection of biological membranes from oxidative stress damage.
Effect of AS on the H2O2 and MDA concentrations and ELR in the leaf, and root of Koshihikari plants subjected to saline and non-saline conditions. Bars represent means ± SE (n = 3) and bars with the same letter are not statistically different (p ≤ 0.05). C = control, S = salt stress, AS = astaxanthin, and ASS = astaxanthin + salt stress
Effect of AS on proline, TP, and TF concentrations under saline and non-saline conditions
The effects of AS on proline, TP, and TF concentrations under saline and non-saline conditions are shown in Fig. 5. Proline concentration in the leaves increased by 28% in salt-stressed plants and further increased by 70% in AS-treated plants. However, the concentration decreased (2.4-fold) in AS-treated plants under non-saline conditions (Fig. 5A). Root proline levels were significantly increased in salt-stressed and salt-stressed plants treated with AS (3.9- and 3.8-fold increases, respectively) compared to those in control plants (Fig. 5B). The TF content in the leaves significantly decreased in salt-stressed (41% decrease), non-stressed AS-treated (9% decrease), and salt-stressed AS-treated plants (25% decrease) (Fig. 5C). The TF content in the roots decreased under salt stress conditions, with non-treated plants having markedly lower contents (41% reduction) than the AS-treated plants (14% reduction) compared with that in the control (Fig. 5D). Salt stress significantly reduced the TP content in the leaves (48% reduction); however, AS restored the content to levels comparable to those in control plants. AS significantly increased the TP content (57% increase) in leaves compared to that in the control under non-saline conditions (Fig. 5E). TP content in the roots increased (2-fold) significantly in salt-stressed plants treated with AS and decreased significantly in plants subjected to other treatments compared with that of the control (Fig. 5F). These results indicated that AS can contribute to the enhanced synthesis of antioxidant compounds that participate in the protection against oxidative damage in leaves and roots under salt stress conditions.
Effect of AS on the concentration of proline (A, B), total flavonoids (C, D) and total phenolics (E, F) in the leaf and root of Koshihikari plants subjected to saline and non-saline conditions. Bars represent means ± SE (n = 3) and bars with the same letter are not statistically different (p ≤ 0.05). C = control, S = salt stress, AS = astaxanthin, and ASS = astaxanthin + salt stress
Effect of AS on the activities of CAT, POD and APX under saline and non-saline conditions
The effects of AS on CAT, POD, and APX activities under saline and non-saline conditions are shown in Fig. 6. CAT activity in the leaves increased significantly under salt stress in untreated plants (2-fold increase) and further increased with the application of AS (3.3-fold increase). However, the CAT activity did not change significantly under non-saline conditions (Fig. 6A). CAT activity in the roots decreased under all treatments (31–46% decrease), with a less pronounced decrease in salt-stressed plants treated with AS (31% decrease) than in plants subjected to other treatments relative to the control (Fig. 6B). The activity of POD in the leaves significantly decreased under salt stress (2.3-fold decrease), but was markedly elevated in AS-treated plants under both saline and non-saline conditions (2.1- and 18-fold increase, respectively) (Fig. 6C). In contrast, the POD activity in the roots did not change significantly across all treatments (Fig. 6D). APX activity in leaves was significantly increased under salt stress in both AS-treated and non-treated plants (4.5- and 2.5-fold increases, respectively; Fig. 6E). APX activity in the roots was only significantly enhanced in salt-stressed plants treated with AS (5.6-fold increase) and remained unaltered in plants subjected to other treatments compared with the control (Fig. 6F). These results indicated that the AS-induced activities of CAT, POD, and APX in the leaves and APX in the roots are important for ROS detoxification, and hence for resistance to oxidative stress, and ultimately, salt stress tolerance.
Effect of AS on the activity of CAT (A, B), POD (C, D) and APX (E, F) in the leaf and root of Koshihikari plants subjected to saline and non-saline conditions. The bars represent means ± SE (n = 3) and bars with the same letter are not statistically different (p ≤ 0.05). C = control, S = salt stress, AS = astaxanthin, and ASS = astaxanthin + salt stress
Effect of AS on the expression of OsBHY, OsNHX1, OsHKT1;5, and OsSOS1 under saline and non-saline conditions
The expression levels of the OsBHY, OsNHX1, OsHKT1;5, and OsSOS1 are presented in Fig. 7. The expression of OsBHY, OsNHX1, and OsSOS1 was upregulated by salt stress in AS-treated and untreated plants. OsBHY expression was similar (2.5-fold change) in salt-stressed and AS-treated plants; however, AS further increased the expression (56% increase) under salt-stress conditions compared with that in non-treated plants under the same conditions (Fig. 7A). The expression of OsNHX1 increased 1.7-fold in salt-treated plants, whereas AS reduced its expression under non-saline (1.3-fold change) and saline (1.4-fold change) conditions (Fig. 7B). OsSOS1 expression was markedly upregulated under salt stress; however, this level decreased by 19% in AS-treated plants grown under salt stress (Fig. 7C). The expression of OsHKT1;5 was downregulated in non-treated plants under salt stress, but was upregulated (1.8-fold) in the presence of AS under the same conditions (Fig. 7D).
Effect of AS on the expression of OsBHY (A) and OsNHX1 (B) in the leaf, and OsHKT1;5 (C) and OsSOS1 (D) in the root of Koshihikari plants subjected to saline and non-saline conditions. Bars represent means ± SE (n = 3). C = control, S = salt stress, AS = astaxanthin, and ASS = astaxanthin + salt stress
Discussion
AS enhances salt stress tolerance in seedlings of Koshihikari Cultivar
In the present study, we evaluated the ameliorative effect of AS (50 µM) on the salt-susceptible rice cultivar, Koshihikari, under salt stress (50 mM NaCl) in hydroponic culture for two weeks. Salt stress significantly reduced the DW of Koshihikari plants, as previously reported (Akter and Oue 2018; Mekawy et al. 2018), providing further support for the susceptibility of this cultivar to salt stress. Interestingly, soaking seeds in AS resulted in enhanced salt stress tolerance of the plants, as growth (DW) was restored to levels comparable to those of the control plants. However, studies on the effects of AS on plant growth under salt stress conditions are scarce. In fact, a study on its effect on strawberry seedlings under salt stress (Zhong et al. 2018) did not report growth parameters, implying that this is the first study to investigate its growth-promoting potential under salt stress.
AS lowers oxidative stress damage in the leaves of Koshihikari by enhancing enzyme and non-enzyme antioxidants
Indicators of oxidative stress damage include the leakage of cellular content due to membrane damage, as revealed by the ELR (Khair and Karim 2015). The higher the ELR, the greater the damage and, consequently, the greater the plant’s susceptibility to salt stress. The second indicator was the concentration of MDA, a product of the peroxidation of membrane lipids caused by ROS (Ahmad et al. 2019). An increase in the concentration of this compound would indicate more damage and is thus a sign of the sensitivity of the plant to stress. The third indicator is the concentration of ROS (usually H2O2) in tissues, where a higher concentration is a sign of a threat to cellular integrity (Yang and Guo 2018). In the present study, the concentrations of H2O2 and MDA in leaf and root tissues and the ELR in leaf tissues were measured to evaluate potential oxidative damage as a contributing factor to the susceptibility of Koshihikari to salt stress. Salt stress significantly increased all three parameters in the leaf tissues. This increase was similar to that previously reported for this cultivar (Mekawy et al. 2018) and other plants, such as eggplant (Assaha et al. 2015b) and sweet pepper (Abdelaal et al. 2019). Furthermore, these increases corresponded to the susceptibility of rice plants to salt stress, indicating that oxidative stress alleviation is important for stress tolerance in Koshihikari. However, priming rice seeds with AS significantly reduced the levels of MDA, H2O2, and ELR under salt stress conditions (Fig. 4), corresponding to the increased stress tolerance of the plants (Fig. 1). This observation ties in with the biological activity of astaxanthin as a ROS scavenger, which is far more potent in ROS detoxification than other potential antioxidants such as α-tocopherol (100 times higher) and β-carotene (10 times higher) (Ambati et al. 2014).
To ascertain how AS contributes to minimizing oxidative stress damage and ROS levels, the antioxidant defense system (enzyme and non-enzyme) was assessed in leaf and root tissues. The non-enzyme antioxidants assessed included proline, TP and TF, whose enhanced accumulation has been associated with high oxidative stress tolerance and hence enhanced salt stress tolerance (Minh et al. 2016; Sarker and Oba 2019; Wutipraditkul et al. 2015). In non-treated plants, salt stress did not affect proline concentration; however it significantly lowered the TF and TP concentrations, indicating that the non-enzyme antioxidant defense system was compromised and ineffective at countering the surging ROS levels under the stress. However, in AS-treated plants the levels of proline, TP, and TF significantly increased compared to non-treated plants under salt stress, clearly indicating that AS is involved in ROS detoxification by restoring the defective antioxidant system induced by salt stress. Proline has been demonstrated to prevent K+ efflux from cells induced by OH• damage (Cuin and Shabala 2007), in addition to its role in osmotic adjustment (Mansour and Ali 2017), indicating that AS may also be implicated in osmoregulation in this plant. The total carotenoid concentration, another potent antioxidant, was observed to not significantly change under the different treatments compared with that in the controls, indicating that total carotenoid may not influence the growth of Koshihikari plants under salt stress. However, to ascertain the role of carotenoids in oxidative stress alleviation, the expression of OsBHY, a gene coding for the production of hydroxylated carotenoids, which are very important in ROS detoxification (Seabra and Pedrosa 2010), was examined. The expression of this gene in the leaves of Koshihikari plants was upregulated by AS and salt stress, and the expression was higher in AS-treated than non-treated plants under salt stress conditions (Fig. 7). Although this enhanced expression did not yield a corresponding increase in the total carotenoid concentration, the abundance of hydroxylated carotenoids may have been significant and thus important for ROS detoxification.
Regarding the enzymatic components of the antioxidant defense system, the activities of CAT, APX, and POD were measured in the roots and leaves. An increase in the activity of these enzymes has been demonstrated to be related to decreased oxidative damage in many plants and, hence, salt stress tolerance, and vice versa (Assaha et al. 2015b; Gharsallah et al. 2016). In the present study, the activities of APX and CAT in the leaves increased in the untreated plants under salt stress, whereas POD activity significantly decreased (Fig. 6). However, the activities of these three enzymes were markedly higher in the AS-treated plants than in the untreated plants under salt stress. APX activity markedly increased in the roots of AS-treated plants under salt stress, whereas POD and CAT activities either remained unchanged or were lower than those of the control (Fig. 6). This finding indicated that the activities of these enzymes in the leaves of untreated plants were insufficient to scavenge excess ROS, leading to oxidative stress damage under salt stress. A lack of correlation between enzyme (SOD, APX, and CAT) activity and oxidative stress tolerance has been previously reported in cowpea plants under salt stress (Cavalcanti et al. 2004). However, the activities of all measured enzymes were enhanced in the presence of AS, similar to observations in the literature relevant to the rice cultivar Nipponbare (Song et al. 2022). This enhanced enzyme activity, combined with the non-enzyme antioxidants observed in the current study, helped lower ROS-induced damage. Generally, the leaves were more affected by ROS than the roots, which showed no signs of damage based on MDA concentration (Fig. 4).
AS may be implicated in controlled Na+ translocation to the shoot
Controlled delivery of Na+ from the root to the shoot is an important characteristic of salt tolerance, particularly in glycophytes (Assaha et al. 2017a). Such controlled delivery has been reported to account for salt stress tolerance in several crops, including huckleberry (Assaha et al. 2015a, b), talinum (Assaha et al. 2017a), tomato (Olias et al. 2009), maize (Zhang et al. 2018), barley (van Bezouw et al. 2019), and wheat (Byrt et al. 2014). However, the inability of plants to control Na+ transport to the shoots, especially to the leaves, is often correlated with salt sensitivity, as observed in some rice cultivars, including Koshihikari (Mekawy et al. 2018) and eggplant (Assaha et al. 2015b). In the present study, the shoot Na+ concentration in untreated plants was greater than that in the shoots of AS-treated plants under salt stress; however, this was not statistically significant (Fig. 2). This finding indicated that AS may be involved in shoot Na+ exclusion, possibly by influencing xylem Na+ retrieval to the surrounding parenchyma cells (Zhu et al. 2016). However, tissues are protected from the damaging effects of cytosolic Na+ through Na+ sequestration into vacuoles via tonoplast NHX1 in plants that accumulate Na+ (Solis et al. 2021). Thus, the upregulation of NHX1 would be an indication of increased vacuolar Na+ sequestration and, hence, enhanced salt stress tolerance in the plant. In contrast, downregulation or lower expression levels would lead to reduced compartmentation and, hence, increased susceptibility to stress due to increased cytosolic Na+ concentrations (Singh et al. 2019). In the present study, the expression of OsNHX1 was upregulated in the untreated and AS-treated plants under salt stress despite a decrease in its expression in AS-treated plants (Fig. 7). As this higher expression in untreated plants corresponds to lower tolerance, NHX1 may not be involved in stress tolerance in this cultivar, whereas reduced Na+ transport in the leaves may be involved.
Reduced transport of Na+ to leaves is controlled by two major transporters: HKT and SOS1. Although SOS1 participates in Na+ efflux from the cells into extracellular compartments or root cells to the external milieu (Oh et al. 2009), HKT localizes at the border of xylem parenchyma cells, and the xylem retrieves Na+ in the transpiration stream of xylem vessels into xylem parenchyma cells (Munns et al. 2012). OsHKT1;5 in rice is known for its role in imparting stress resistance to tolerant rice cultivars (Cotsaftis et al. 2012; Horie et al. 2012). Accordingly, downregulation of the genes encoding these two transporters leads to the uncontrolled delivery of toxic levels of Na+ to photosynthetic tissues, which may affect biomass production and, hence, susceptibility. In the present study, the expression of OsSOS1 is induced in both treated and untreated plants under salt stress, despite its lower expression in the AS-treated plants. As the Na+ concentration in the leaves was higher in non-treated plants, this enhanced OsSOS1 expression may imply two possibilities: (1) the expression was insufficient to recycle absorbed Na+ to the soil, and (2) the induced OsSOS1 may instead be implicated in xylem Na+ loading for translocation to the shoot (Katschnig et al. 2015). Conversely, lower expression in AS-treated plants indicated lower xylem loading and, coupled with retrieval from the xylem owing to the upregulation of OsHKT1;5, translocation to the shoot was restricted to tolerable levels that would not compromise the growth of Koshihikari plants under salt stress. In addition, a relatively weak correlation between OsSOS1 expression in the roots and Na+ efflux has been established, with high expression corresponding to lower Na+ efflux in Koshihikari, as opposed to a strong positive correlation in tolerant cultivars (Liu et al. 2019). The same authors also noticed damaged pericycle cells (the major site for Na+ sequestration in the root), which are believed to contribute to increased Na+ accumulation in the shoot, leading to loss of tolerance through loss of root tissue tolerance.
Normally, reduced root-to-shoot Na+ transport is associated with increased K+ accumulation in shoots under salt stress (Hauser and Horie 2010), owing to the competitive uptake and transport of Na+ over K+ under salt stress (Assaha et al. 2017b). Consequently, increased shoot K+ accumulation and reduced leaf Na+/K+ ratios, especially low cytosolic Na+/K+ ratios, have become important salt tolerance traits (Assaha et al. 2017b). In the present study, shoot K+ levels declined under salt stress and remained low in AS-treated plants, indicating that AS was not involved in K+ transport to the shoots. Reduced K+ accumulation in Koshihikari under salt stress has been observed and attributed to high K+ efflux from roots induced by ROS, as well as the reduced expression of K+ channels such as OsAKT1 (Liu et al. 2019). However, as shoot Na+ levels declined in AS-treated plants, the shoot Na+/K+ ratio was markedly lower in AS-treated plants than in untreated plants, suggesting its importance in salt stress tolerance.
Conclusions
This study aimed to evaluate the role of AS in salt stress alleviation in the sensitive rice cultivar Koshihikari. The results clearly revealed the importance of AS in reducing oxidative stress damage, as ROS levels, in terms of H2O2, decreased with a corresponding decline in ELR and MDA (indicators of oxidative damage). In addition, AS-induced OsHKT1;5 upregulation under salt stress appeared to play a critical role in regulating the Na+/K+ ratio under salt stress, which is an important stress tolerance trait. Thus, AS is important for alleviating oxidative stress damage and maintaining a normal Na+ and K+ homeostatic balance, resulting in enhanced salt stress tolerance in Koshihikari. Hence, the AS (50 µM) application is a potential alternative for enhancing Koshihikari production in salt-affected soils. However, further investigations are needed to clarify the underlying molecular mechanism of AS involvement in the antioxidant defense system as well as in the regulation of Na+ uptake and translocation to the shoot.
References
Abdelaal KA, El-Maghraby LM, Elansary H, Hafez YM, Ibrahim EI, El-Banna M, El-Esawi M, Elkelish A (2019) Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 10(1):26. https://doi.org/10.3390/agronomy10010026
Ahmad R, Hussain S, Anjum MA, Khalid MF, Saqib M, Zakir I, Hassan A, Fahad S, Ahmad S (2019) Oxidative stress and antioxidant defense mechanisms in plants under salt stress. Plant abiotic stress tolerance. Springer, Heidelberg, pp 191–205. https://doi.org/10.1007/978-3-030-06118-0_8
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2(4):875–877. https://doi.org/10.1038/nprot.2007.102
Akter M, Oue H (2018) Effect of saline irrigation on accumulation of Na+, K+, Ca2+, and Mg2+ ions in rice plants. Agriculture 8(10):164. https://doi.org/10.3390/agriculture8100164
Ambati RR, Phang SM, Ravi S, Aswathanarayana RG (2014) Astaxanthin: sources, extraction, stability, biological activities and its commercial applications–a review. Mar Drugs 12(1):128–152. https://doi.org/10.3390/md12010128
Assaha DV, Mekawy AM, Ueda A, Saneoka H (2015a) Salinity-induced expression of HKT may be crucial for Na+ exclusion in the leaf blade of huckleberry (Solanum scabrum Mill.), but not of eggplant (Solanum melongena L). Biochem Biophys Res Commun 460(2):416–421. https://doi.org/10.1016/j.bbrc.2015.03.048
Assaha DVM, Liu LY, Mekawy AMM, Ueda A, Nagaoka T, Saneoka H (2015b) Effect of salt stress on Na+ accumulation, antioxidant enzyme activities and activity of cell wall peroxidase of huckleberry (Solanum scabrum) and eggplant (Solanum melongena). Int J Agric Biol 17(6):1149–1156. https://doi.org/10.17957/Ijab/15.0052
Assaha DVM, Mekawy AMM, Liu L, Noori MS, Kokulan KS, Ueda A, Nagaoka T, Saneoka H (2017a) Na+ retention in the root is a key adaptive mechanism to low and high salinity in the glycophyte, Talinum paniculatum (Jacq.) Gaertn. (Portulacaceae). J Agron Crop Sci 203(1):56–67. https://doi.org/10.1111/jac.12184
Assaha DVM, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW (2017b) The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509. https://doi.org/10.3389/fphys.2017.00509
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207. https://doi.org/10.1007/Bf00018060
Byrt CS, Xu B, Krishnan M, Lightfoot DJ, Athman A, Jacobs AK, Watson-Haigh NS, Plett D, Munns R, Tester M, Gilliham M (2014) The Na+ transporter, TaHKT1;5-D, limits shoot Na+ accumulation in bread wheat. Plant J 80(3):516–526. https://doi.org/10.1111/tpj.12651
Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viégas RA, Silveira JAG (2004) Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol 163(3):563–571. https://doi.org/10.1111/j.1469-8137.2004.01139.x
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775. https://doi.org/10.1016/S0076-6879(55)02300-8
Chuamnakthong S, Nampei M, Ueda A (2019) Characterization of Na+ exclusion mechanism in rice under saline-alkaline stress conditions. Plant Sci 287:110171. https://doi.org/10.1016/j.plantsci.2019.110171
Cotsaftis O, Plett D, Shirley N, Tester M, Hrmova M (2012) A two-staged model of Na+ exclusion in rice explained by 3D modeling of HKT transporters and alternative splicing. PLoS ONE 7(7):e39865. https://doi.org/10.1371/journal.pone.0039865
Cuin TA, Shabala S (2007) Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ 30(7):875–885. https://doi.org/10.1111/j.1365-3040.2007.01674.x
Fang S, Hou X, Liang X (2021) Response mechanisms of plants under saline-alkali stress. Front Plant Sci 12:667458. https://doi.org/10.3389/fpls.2021.667458
Fujimaki S, Maruyama T, Suzui N, Kawachi N, Miwa E, Higuchi K (2015) Base to tip and long-distance transport of sodium in the root of Common Reed [Phragmites australis (Cav.) Trin. Ex Steud.] At steady state under constant high-salt conditions. Plant Cell Physiol 56(5):943–950. https://doi.org/10.1093/pcp/pcv021
Gharsallah C, Fakhfakh H, Grubb D, Gorsane F (2016) Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 8:plw055. https://doi.org/10.1093/aobpla/plw055
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Hauser F, Horie T (2010) A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ 33(4):552–565. https://doi.org/10.1111/j.1365-3040.2009.02056.x
Himabindu Y, Chakradhar T, Reddy MC, Kanygin A, Redding KE, Chandrasekhar T (2016) Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ Exp Bot 124:39–63. https://doi.org/10.1016/j.envexpbot.2015.11.010
Horie T, Karahara I, Katsuhara M (2012) Salinity tolerance mechanisms in glycophytes: an overview with the central focus on rice plants. Rice 5(1):11. https://doi.org/10.1186/1939-8433-5-11
Katschnig D, Bliek T, Rozema J, Schat H (2015) Constitutive high-level SOS1 expression and absence of HKT1;1 expression in the salt-accumulating halophyte Salicornia Dolichostachya. Plant Sci 234:144–154. https://doi.org/10.1016/j.plantsci.2015.02.011
Khair TSAB, Karim MF (2015) The use of electrolyte leakage procedure in assessing heat and salt tolerance of Ruzaiz date palm (Phoenix dactylifera L.) cultivar regenerated by tissue culture and offshoots and treatments to alleviate the stressful injury. J Hortic for 7(4):104–111. https://doi.org/10.5897/jhf2014.0378
Kobayashi A, Hori K, Yamamoto T, Yano M (2018) Koshihikari: a premium short-grain rice cultivar - its expansion and breeding in Japan. Rice 11(1):1–12. https://doi.org/10.1186/s12284-018-0207-4
Liu J, Shabala S, Shabala L, Zhou M, Meinke H, Venkataraman G, Chen Z, Zeng F, Zhao Q (2019) Tissue-specific regulation of Na+ and K+ transporters explains genotypic differences in salinity stress tolerance in rice. Front Plant Sci 10:1361. https://doi.org/10.3389/fpls.2019.01361
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Mangu VR, Ratnasekera D, Yabes JC, Wing R, Baisakh N (2019) Functional screening of genes from a halophyte wild rice relative in model identifies candidate genes involved in salt tolerance. Curr Plant Biol 18:100107. https://doi.org/10.1016/j.cpb.2019.100107
Mansour MMF, Ali EF (2017) Evaluation of proline functions in saline conditions. Phytochem 140:52–68. https://doi.org/10.1016/j.phytochem.2017.04.016
Mekawy AM, Assaha DV, Yahagi H, Tada Y, Ueda A, Saneoka H (2015) Growth, physiological adaptation, and gene expression analysis of two Egyptian rice cultivars under salt stress. Plant Physiol Biochem 87:17–25. https://doi.org/10.1016/j.plaphy.2014.12.007
Mekawy AMM, Abdelaziz MN, Ueda A (2018) Apigenin pretreatment enhances growth and salinity tolerance of rice seedlings. Plant Physiol Biochem 130:94–104. https://doi.org/10.1016/j.plaphy.2018.06.036
Memari-Tabrizi EF, Dokhanieh AY, Babashpour-Asl M (2021) Foliar-applied silicon nanoparticles mitigate cadmium stress through physio-chemical changes to improve growth, antioxidant capacity, and essential oil profile of Summer Savory (Satureja hortensis L). Plant Physiol Biochem 165:71–79. https://doi.org/10.1016/j.plaphy.2021.04.040
Minh LT, Khang DT, Ha PTT, Tuyen PT, Minh TN, Quan NV, Xuan TD (2016) Effects of salinity stress on growth and phenolics of rice (Oryza sativa L). Int Lett Nat Sci 57:1–10. https://doi.org/10.18052/www.scipress.com/ILNS.57.1
Mitsuya S, Murakami N, Sato T, Kazama T, Toriyama K, Skoulding NS, Kano-Nakata M, Yamauchi A (2019) Evaluation of rice grain yield and yield components of Nona Bokra chromosome segment substitution lines with the genetic background of Koshihikari, in a saline paddy field. AoB Plants 11(5):plz040. https://doi.org/10.1093/aobpla/plz040
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Munns R, James RA, Xu B, Athman A, Conn SJ, Jordans C, Byrt CS, Hare RA, Tyerman SD, Tester M, Plett D, Gilliham M (2012) Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat Biotechnol 30(4):360–364. https://doi.org/10.1038/nbt.2120
Munns R, Day DA, Fricke W, Watt M, Arsova B, Barkla BJ, Bose J, Byrt CS, Chen ZH, Foster KJ, Gilliham M, Henderson SW, Jenkins CLD, Kronzucker HJ, Miklavcic SJ, Plett D, Roy SJ, Shabala S, Shelden MC, Soole KL, Taylor NL, Tester M, Wege S, Wegner LH, Tyerman SD (2020a) Energy costs of salt tolerance in crop plants. New Phytol 225(3):1072–1090. https://doi.org/10.1111/nph.15864
Munns R, Passioura JB, Colmer TD, Byrt CS (2020b) Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol 225(3):1091–1096. https://doi.org/10.1111/nph.15862
Nampei M, Jiadkong K, Chuamnakthong S, Wangsawang T, Sreewongchai T, Ueda A (2021) Different rhizospheric pH conditions affect nutrient accumulations in rice under salinity stress. Plants (Basel) 10(7):1295. https://doi.org/10.3390/plants10071295
Oh DH, Leidi E, Zhang Q, Hwang SM, Li Y, Quintero FJ, Jiang X, D’Urzo MP, Lee SY, Zhao Y, Bahk JD, Bressan RA, Yun DJ, Pardo JM, Bohnert HJ (2009) Loss of halophytism by interference with SOS1 expression. Plant Physiol 151(1):210–222. https://doi.org/10.1104/pp.109.137802
Olias R, Eljakaoui Z, Li J, De Morales PA, Marin-Manzano MC, Pardo JM, Belver A (2009) The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ 32(7):904–916. https://doi.org/10.1111/j.1365-3040.2009.01971.x
Özbeyli D, Gürler EB, Buzcu H, Çilingir-Kaya OT, Çam ME, Yüksel M (2020) Astaxanthin alleviates oxidative damage in acute pancreatitis via direct antioxidant mechanisms. Turk J Gastroenterol 31(10):706–712. https://doi.org/10.5152/tjg.2020.19520
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta - Bioenerg 975(3):384–394. https://doi.org/10.1016/s0005-2728(89)80347-0
Prusty MR, Kim SR, Vinarao R, Entila F, Egdane J, Diaz MGQ, Jena KK (2018) Newly identified wild rice accessions conferring high salt tolerance might use a tissue tolerance mechanism in leaf. Front Plant Sci 9:417. https://doi.org/10.3389/fpls.2018.00417
Röös E, Bajželj B, Smith P, Patel M, Little D, Garnett T (2017) Greedy or needy? Land use and climate impacts of food in 2050 under different livestock futures. Glob Environ Change 47:1–12. https://doi.org/10.1016/j.gloenvcha.2017.09.001
Sarker U, Oba S (2019) Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J Sci Food Agric 99(5):2275–2284. https://doi.org/10.1002/jsfa.9423
Seabra LMJ, Pedrosa LFC (2010) Astaxanthin: structural and functional aspects. Rev Nutr 23(6):1041–1050. https://doi.org/10.1590/S1415-52732010000600010
Shabala S, Bose J, Hedrich R (2014) Salt bladders: do they matter? Trends Plant Sci 19(11):687–691. https://doi.org/10.1016/j.tplants.2014.09.001
Shams M, Ekinci M, Ors S, Turan M, Agar G, Kul R, Yildirim E (2019) Nitric oxide mitigates salt stress effects of pepper seedlings by altering nutrient uptake, enzyme activity and osmolyte accumulation. Physiol Mol Biol Plants 25(5):1149–1161. https://doi.org/10.1007/s12298-019-00692-2
Singh J, Singh V, Vineeth TV, Kumar P, Kumar N, Sharma PC (2019) Differential response of Indian mustard (Brassica juncea L., Czern and Coss) under salinity: photosynthetic traits and gene expression. Physiol Mol Biol Plants 25(1):71–83. https://doi.org/10.1007/s12298-018-0631-3
Solis CA, Yong MT, Venkataraman G, Milham P, Zhou M, Shabala L, Holford P, Shabala S, Chen ZH (2021) Sodium sequestration confers salinity tolerance in an ancestral wild rice. Physiol Plant 172(3):1594–1608. https://doi.org/10.1111/ppl.13352
Song Y, Zheng C, Basnet R, Li S, Chen J, Jiang M (2022) Astaxanthin synthesized gold nanoparticles enhance salt stress tolerance in rice by enhancing tetrapyrrole biosynthesis and scavenging reactive oxygen species in vitro. Plant Stress 6:100122. https://doi.org/10.1016/j.stress.2022.100122
Sriskantharajah K, Osumi S, Chuamnakthong S, Nampei M, Amas JC, Gregorio GB, Ueda A (2020) Contribution of two different Na+ transport systems to acquired salinity tolerance in rice. Plant Sci 297:110517. https://doi.org/10.1016/j.plantsci.2020.110517
Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K (2002) The heterotrimeric G protein α subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA 99(20):13307–13312. https://doi.org/10.1073/pnas.192244099
Tahjib-Ul-Arif M, Abu Sayed M, Islam MM, Siddiqui MN, Begum SN, Hossain MA (2018) Screening of rice landraces (Oryza sativa L.) for seedling stage salinity tolerance using morpho-physiological and molecular markers. Acta Physiol Plant 40(4):70. https://doi.org/10.1007/s11738-018-2645-4
Takagi H, Yamada S (2013) Roles of enzymes in anti-oxidative response system on three species of chenopodiaceous halophytes under NaCl-stress condition. Soil Sci Plant Nutr 59(4):603–611. https://doi.org/10.1080/00380768.2013.809600
Toft KH (2012) GMOs and global justice: applying global justice theory to the case of genetically modified crops and food. J Agric Environ Ethics 25(2):223–237. https://doi.org/10.1007/s10806-010-9295-x
van Bezouw R, Janssen EM, Ashrafuzzaman M, Ghahramanzadeh R, Kilian B, Graner A, Visser RGF, van der Linden CG (2019) Shoot sodium exclusion in salt stressed barley (Hordeum vulgare L.) is determined by allele specific increased expression of HKT1;5. J Plant Physiol 241:153029. https://doi.org/10.1016/j.jplph.2019.153029
Wellburn AR (1994) The spectral determination of chlorophyll-a and chlorophhyll-b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144(3):307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Wutipraditkul N, Wongwean P, Buaboocha T (2015) Alleviation of salt-induced oxidative stress in rice seedlings by proline and/or glycinebetaine. Biol Plant 59(3):547–553. https://doi.org/10.1007/s10535-015-0523-0
Yang Y, Guo Y (2018) Unraveling salt stress signaling in plants. J Integr Plant Biol 60(9):796–804. https://doi.org/10.1111/jipb.12689
Yang Z, Li JL, Liu LN, Xie Q, Sui N (2019) Photosynthetic regulation under salt stress and salt-tolerance mechanism of Sweet Sorghum. Front Plant Sci 10:1722. https://doi.org/10.3389/fpls.2019.01722
Zhang M, Cao Y, Wang Z, Wang ZQ, Shi J, Liang X, Song W, Chen Q, Lai J, Jiang C (2018) A retrotransposon in an HKT1 family sodium transporter causes variation of leaf Na+ exclusion and salt tolerance in maize. New Phytol 217(3):1161–1176. https://doi.org/10.1111/nph.14882
Zhong Y, Luo Y, Ge C, Mo Q, Luo S Effect of astaxanthin on the growth and resistance of strawberry seedling under salt stress. In: 2017 3rd International Forum on Energy, Environment Science and Materials (IFEESM 2017), 2018. Atlantis Press, pp 2139–2143. https://doi.org/10.2991/ifeesm-17.2018.385
Zhu M, Shabala L, Cuin TA, Huang X, Zhou M, Munns R, Shabala S (2016) Nax loci affect SOS1-like Na+/H+ exchanger expression and activity in wheat. J Exp Bot 67(3):835–844. https://doi.org/10.1093/jxb/erv493
Zhu Q, Zeng D, Yu S, Cui C, Li J, Li H, Chen J, Zhang R, Zhao X, Chen L, Liu YG (2018) From golden rice to aSTARice: Bioengineering astaxanthin biosynthesis in rice endosperm. Mol Plant 11(12):1440–1448. https://doi.org/10.1016/j.molp.2018.09.007
Acknowledgements
This research was supported by JSPS KAKENHI Grant Number 20KK0129 to AU. AMMM extends his appreciation to the Academy of Scientific Research and Technology (ASRT, Egypt) for funding the Graduation Projects conducted at Laboratory of Plant Nutritional Physiology, Minia University, Egypt.
Funding
Open Access funding provided by Hiroshima University.
Author information
Authors and Affiliations
Contributions
The authors have made the following declarations about their contributions: Conceived and designed the experiments: AMMM, AU. Performed the experiments: AMMM, LJ. Analyzed the data: AMMM, LJ, and DVMA. Wrote the paper: AMMM, DVMA, and AU.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no conflicts of interest.
Additional information
Communicated by Dezhi Wu.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mekawy, A.M.M., Assaha, D.V.M., Li, J. et al. Astaxanthin application enhances salinity tolerance in rice seedlings by abating oxidative stress effects and enhancing Na+/K+ homeostatic balance. Plant Growth Regul (2024). https://doi.org/10.1007/s10725-024-01132-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10725-024-01132-2