Abstract
The main objectives of the current study were synthesis and characterization of zinc oxide nanoparticles (ZnONPs) using the aqueous leaf extract of Guazuma ulmifolia (G. ulmifolia) plant and assessing its antimicrobial and antioxidant potential. The nanoparticles were characterized using Ultraviolet Visible (UV–Vis), Fourier Transform Infrared (FTIR) Spectroscopy, X-ray diffraction (XRD), Energy-dispersive X-ray (EDS) and Scanning electron microscopy (SEM). ZnONPs exhibited a maximum peak at 342 nm in the UV–Vis spectrum, indicating their absorption properties. FTIR analysis confirmed the presence of functional groups, such as OH and COOH which likely contributed to the stability of the nanoparticles. The XRD analysis confirmed the spherical structure of the nanoparticles as indicated by distinct diffraction peaks corresponding to the lattice planes of the ZnO standard. The EDS analysis confirmed the presence of Zn, C and O in the ZnONPs. SEM analysis provided insights into the nanoparticles size, shape and surface morphology, showing an average size between 19 and 41 nm. Furthermore, the synthesized ZnONPs demonstrated excellent antioxidant and antibacterial activities against various bacteria and Candida species. This study highlights the promising potential of ZnONPs in diverse fields, including biomedicine, due to their low toxicity, economic viability and beneficial properties. It is crucial to conduct further in vivo investigations in order to determine the safety, effectiveness and compatibility of G. ulmifolia ZnONPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, ZnONPs have received a lot of attention due to their diverse applications. They are widely used in diverse fields, such as food, agriculture, biomedical, dentistry and pharmaceuticals. They exhibit antibacterial, antifungal and anticancer activities, making them suitable for use in food packaging, medical imaging, drug delivery and cancer treatment [1,2,3,4]. Rahman et al. studied the influence of Mg and Cu dual-doping on phytogenically ZnONPs. Dual-doping enhances light-induced antibacterial and radical scavenging activities, showing potential for antibacterial treatments and antioxidant therapies [3, 4]. Additionally, ZnONPs have been found to be biocompatible, low in toxicity, and cost-effective, further enhancing their attractiveness for its uses [2, 5, 6]. In dentistry, these nanoparticles have been utilized for restorative dentistry, endodontics, periodontics and oral medicine, among other areas, owing to their unique optical, mechanical and regenerative properties [7]. The synthesis, characterization and modification of nanoparticles have been extensively studied and their potential in various fields continues to be explored. The growing body of research supports the promising and versatile nature of ZnONPs for numerous practical applications [3, 4, 6,7,8,9,10]. In earlier studies, ZnONPs synthesized with the leaf extract of Ziziphus mauritiana Lam. exhibited properties [3, 4, 8].
The mechanism of formation of ZnONPs can be elucidated by different methods. One proposed mechanism involves the hydrolysis of zinc precursors, formation of ZnONPs. For instance, in the polyol chemistry method, the reaction between zinc acetate dihydrate and diethylene glycol or triethylene glycol leads to esterification, forming zinc hydroxide, which dehydrates to produce ZnONPs [11]. Additionally, the mechanochemical method involves the milling process of zinc precursors, resulting in the formation of ZnONPs through subsequent calcination [8, 9, 12]. Furthermore, the green synthesis approach, using plant extracts, has been shown to facilitate the formation of ZnONPs through bio-reduction and capping mechanisms [1, 2]. These mechanisms demonstrate the diverse pathways through which ZnONPs can be formed, each with their unique chemical and physical processes [13].
Due to the harsh traditional chemical and physical processes that are linked to global environmental problems. It has become more vital [14,15,16,17]. Due to its innovative nature and broad range of applications in nearly all scientific and technological domains, including the biological sciences, nanotechnology has garnered increased attention [18, 19]. Conventionally, a range of physical and chemical techniques are employed to produce nano particles, which can be expensive and pose risks to both the environment and human well-being [17,18,19,20,21,22]. Although they enable the large-scale creation of stable nanoparticles in a variety of forms and sizes, they possess several disadvantages. On the other hand, plants are the most popular green, simple, and environmentally friendly method for creating nanoparticles [23, 24]. Phytochemicals that function as both capping or stabilizing and reducing agents can be found in plant extracts [25,26,27,28].
There is a need for alternative environmentally friendly methods to synthesize nanoparticles, considering the increasing need to minimize waste and accomplish sustainable development goals. This can be achieved by applying the fundamental principles of green chemistry [29, 30].
Our aim is to create echo-friendly ZnONPs, characterized with UV–Vis, FTIR, SEM, EDX, XRD methods and screen the synthesized ZnONPs as antioxidants and antimicrobials.
2 Experimental
2.1 Materials
Fresh leaves of G. ulmifolia were procured from a place near the college campus. Zinc acetate (SRL, India, 99.5%), nutrient broth (Hi media, India), agar power (Hi media, India) and all the constituents for preparation of media were obtained from Hi Media Laboratories. The chemicals used were of high purity. UV visible spectrum was recorded using spectrophotometer (UV Shimadzu 1700).
10 g of fresh leaves were taken and cleaned using distilled water, then finely chopped and grinded using mortar pestle and in 250 mL beaker the grinded leaves were added to 100 mL of distilled water and boiled using Bunsen burner for 15 min and cooled. The filtrate was allowed to centrifuge at 8000 rpm for 10 min and the supernatant was stored [3, 4].
2.2 Synthesis of ZnONPs
Zinc acetate [Zn(CH3COO)2.2H2O] solution was prepared by dissolving 2.195 g of zinc acetate (0.1 M) in 100 mL of distilled water and for complete solubilization. It was kept in magnetic stirrer for 1 h. Following this 20 mL of leaf extract was added drop wise to 80 mL of zinc acetate solution and is stirred continuously and stirring should be kept continuous. NaOH (2 M) was freshly prepared and was added drop wise to the solution with mild stirring and the pH was adjusted to 10. After this the solution was kept in water bath at 50° C for 1 h and stirring of reaction mixture was continued for 2 h using magnetic stirrer. A white crystalline precipitate was obtained and then was allowed to wash repeated times using H2O and checked till pH 7. Using UV Vis spectrophotometry, the precipitate absorbance was measured between 200 and 600 nm to ensure the formation of zinc oxide nanoparticles. Then the precipitate was air dried in oven at 50 °C and the sample was stored in powder form for further process [8,9,10, 31, 32].
2.3 Effect of pH
The pH of the green synthesized solution is one of the factors influencing the synthesis of ZnONPs. The pH was adjusted to 6, 8, 9, and 10 using HCl (1 M) and NaOH (1 M). The absorbance was then measured using a double beam UV–Vis spectrophotometer in the range of 200–800 nm. Zinc acetate (0.1 M) solution was prepared, 40 mL of this solution, 10 mL of plant leaves extract was added drop wise, and 10 mL of this mixture was transferred to five test tubes and by adding NaOH, each test tubes were set to different pH values as 6, 8, 9, 10. The test tubes were heated in the water bath at 50 °C.
2.4 Effect of temperature
Temperature has a crucial role in the synthesis of ZnONPs. The synthesis of a representative sample was conducted at temperatures of 50, 60 and 70 °C maintaining an optimal pH level. The suspensions UV–Vis absorption spectra were measured at various temperature ranges.
2.5 UV–Visible studies
With the help of UV–Vis Spectrophotometer, (Model- UV 9600A, Kyoto, Japan), we investigated the absorption characteristics of the produced ZnONPs. Finding the ZnONPs' absorption maxima in the 300–600 nm wavelength range was the aim of the analysis. The blank was created from deionized water from Milli-Q purification equipment. A graph was created to show the absorption behavior of the ZnONPs [33, 34].
2.6 FTIR studies
Using a Thermo Scientific™ Nicolet iSTM50 FTIR Spectrometer, FTIR was examined to know the potential functional groups present in the leaf extract. The 400–4000 cm–1 range was the focus of FTIR analysis.
2.7 SEM—EDS studies
The surface morphology & elements contained in the green synthesized ZnONPs were studied by SEM–EDS spectroscopy (model: JSM-IT500 JEOL, USA). Prior to being affixed onto a sample holder, the sample was adhered to a piece of double-sided adhesive tape. A 10 µm coating of gold was deposited onto the sample under vacuum conditions. The sample, which was coated using a sputtering technique, was examined by scanning it with an accelerating voltage of 5 kV.
2.8 XRD studies
XRD was performed to measure the X-ray intensities and scattering angles that escape a material in response to input X-ray radiation [35, 36]. The crystalline structure of ZnONPs was investigated through the use of a Rigaku SmartLab SE X-ray diffractometer, Japan. The apparatus used in this study was equipped with a copper K-alpha radiation source with a wavelength of 1.54 Å. It was operated at an applied voltage of 40 kV and a current of 30 mA. The diffractogram was acquired by scanning at a rate of 5° min−1 over the range of 1–100 Å.
The average size (D) of the nanocrystals calculated as:
2.9 Qualitative phytochemical analysis of G. ulmifolia leaf extract
Aqueous extract of G. ulmifolia was subjected to phytochemical analysis using the previously described methods [35] for the determination of flavonoids, alkaloids, tannins, etc. [36].
3 Applications
3.1 Antimicrobial activity
Antimicrobial potential of the synthesized ZnONPs was determined using agar well diffusion. Microbial cultures that were 24 h old were utilized for the purpose of agar disc diffusion antibiotic susceptibility assay. 0.1 mL of microbial inoculum was uniformly distributed throughout the entire agar plate. surface to inoculate it. An aseptic procedure was used to create a hole with a diameter of 8 mm using a sterile tip. A solution containing 10 mg of ZnONPs was prepared by dissolving them in 1 mL of distilled water. Then, a small volume of 50 μl of this solution was added to the well and incubated in a bacteriological incubator [3, 4, 6].
3.2 Antioxidant activity
The antioxidant study of ZnONPs was assessed by standard method. Different concentrations of ZnONPs and leaf extract were prepared separately in test tubes 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was introduced into each and allowed to incubate in darkness for a duration of 20 min. UV–Vis Spectrometer used to measure absorbance at 510 nm [3, 4].
4 Results and discussion
4.1 Phytochemical tests
Phytochemical analysis of G. ulmifolia showed the presence of alkaloids, proteins, phenols, saponins, and flavonoids (Table 1). These compounds have various biological activities and potential health benefits. However, glycosides, anthraquinones, and tannins were not detected in the sample. Further research is needed to explore the specific compounds present and their potential applications. The traditional applications of Guazoma have been scientifically validated through in-vitro and in-vivo investigations, which have linked its bioactivities to its phytochemical composition and beneficial qualities.
4.2 Effect of change in pH
In the experiment to find the optimum pH, colour of the solution turned from pale yellow to yellow coloured (Table 2) precipitate which showed the presence of ZnONPs.
This table consolidates the information about the effects of changing pH and incubation temperature on the synthesis of ZnONPs using plant leaf extracts. It highlights the optimal conditions (pH 10 at temp. 60°C) for the synthesis process, as shown by the colour change to yellow and the characteristic peak at 342 nm.
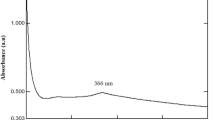
The change from pale yellow colour to yellow coloured precipitate was the confirmatory sign for nanoparticle synthesis. The characteristic peak value for nanoparticle synthesis at pH 10 was observed at 342 nm at maximum (Fig. 1). It was observed that at this pH it was shown the reduction of ZnO and synthesis of ZnONPs.
4.3 Effect of change in incubation temperature
Temperature conditions ranging from 50 to 70 °C were utilized to incubate ZnONPs. The incubation process involved the combination of 2 mL of leaves extract and 2 mL of zinc acetate (0.1 M) solution, with the pH set at 10. The resulting synthesis of ZnONPs was then analyzed using a UV spectrometer. Notably, a distinct and concentrated peak (Table 2) was observed at the optimal temperature of 60 °C (Fig. 2).
5 Characterization
5.1 UV–Vis analysis of ZnONPs
ZnONPs production was monitored by taking absorption spectra between 300 and 800 nm. After a 24 h incubation period in a darkroom, UV–visible spectroscopic analysis at 342 nm confirmed successful synthesis of ZnONPs (Fig. 3). This analysis provides evidence for the presence of ZnONPs in the solution, which allowed for further characterization and potential applications in various fields.
5.2 FTIR Analysis
The FTIR analysis of synthesized ZnONPs were carried out to analyses the functional groups present in ZnONPs. The FTIR analysis of synthesized ZnONPs shows many peaks at 400–4000 cm−1. Peaks were obtained at 1603 cm−1 correspond to Zn–O stretching, at 3354 cm−1 indicates the OH vibration and 1123 cm−1, CO stretching in amino acid. Peak at 1506 cm−1 indicates aromatic C = C stretching, 936 cm−1 describes CH bonding (Fig. 4(a, b)).
5.3 SEM Study
To observe the ZnONPs size, shape, and surface, SEM analysis was done. SEMVEGA 3 TESCAN model was utilized to ascertain the ZnONPs form across various magnification levels. The spherical morphology and aggregated NPs of the ZnONPs were revealed by the SEM magnification images. SEM pictures of biosynthesized ZnONPs in 2000, 5000, 12,000 and 13,000 magnifications (Fig. 5). The zinc nanoparticles were found to have an average size ranging from 19 to 41 nm by measuring in Image J software and they exhibited a spherical shape.
5.4 EDS analysis
The presence of metallic zinc acetate was established by EDS analysis of the synthesized ZnONPs. Zn, C and O presence was confirmed in the ZnONPs. Zinc had three peaks in the EDS spectrum, while oxygen and carbon only have one peak (Fig. 6). In the EDS study, peaks caused by the SPR of zinc oxide NPs are clearly visible. The trace amount of oxygen indicates the presence of plant phytochemicals in metal ion reduction, capping agents of ZnONPs synthesis. The ZnONPs synthesis was verified by the presence of zinc oxide ions at 10 keV in the elemental biosynthesized ZnO utilizing G. ulmifolia extract.
5.5 XRD study
The synthesized ZnONPs, the XRD pattern clearly displays the crystal image of the nanoparticles. At 2θ values of 31.46, 34.29, 36.33, 47.51, 58.50, 62.84, 67.79, and 76.83 degrees, we could see the distinct diffraction peaks. Diffraction lattice planes 100, 002,101, 102,110,103, 112 & 202 are corresponding peaks (Fig. 7), and they verify that the produced nanoparticles have a spherical structure having a range of 19 to 41 nm (Table 3), this pattern matches the standard peaks (JCPDS 36–1451), which also matches with the values previously reported [37, 38].
5.6 Antimicrobial activity
The agar well diffusion method is a widely accepted approach to assess the antibacterial properties of plants extracts. By diffusing into the agar medium, the antimicrobial agent can effectively hinder the growth of the tested microbial strains [4, 6, 8, 39,40,41]. The results of the present study can be seen in Table 4 and Figs. 8 and 9. The experiment employed two gram +ve, three-gram -ve bacteria, for the bacteria strains, ampicillin was used as a standard antibiotic. For fungal strain, Fluconazole was used as the standard. When tested against E. coli, ZnONPs exhibited remarkable antibacterial effectiveness, while other strains showed slightly lower activity. Zinc oxide nanoparticles possess a multifaceted antibacterial mechanism that involves various impacts on bacterial cells, interactions at a physicochemical level, and mechanisms of cellular absorption. Understanding these mechanisms is essential for developing effective antibacterial treatments and strategically designing antimicrobial medicines using nanomaterials. Developing effective antibacterial treatments necessitates a deep comprehension of these complex systems. With the expertise of a pharmacologist, it becomes feasible to strategically develop antimicrobial medications using nanomaterials, harnessing their unique properties to precisely combat microbial infections. Utilizing zinc nanoparticles in antimicrobial techniques shows promise in enhancing the effectiveness of existing treatments and tackling the problem of antibiotic resistance.
The data is presented in sets of three replicates (n = 3), with mean ± SEM indicated. The STD used for fungi was Fluconazole, while for bacteria it was Ampicillin.
5.7 Antioxidant activity
The antioxidant activity of synthesized ZnONPs was carried out using standard method (Fig. 10). The ZnONPs showed promising antioxidant activity, consistent with previous research that investigated the antioxidant properties of various plant extracts for nano preparations [4, 6, 8, 42,43,44,45,46,47].
By assessing the DPPH scavenging activity of the two substances at different doses (0.125, 0.25, 0.5, and 1.0 mg/mL), the bar graph shows the antioxidant potential of both the aqueous plant extract and the ZnONPs. One well-known technique for evaluating antioxidant potential is the DPPH assay, which measures a substance's strength as an antioxidant by measuring its capacity to neutralise DPPH free radicals. According to this analysis, the DPPH scavenging ability of the aqueous plant extract increases with concentration, peaking at 1 mg/mL, and this indicates increased antioxidant activity. This shows that the plant extract's ability to stabilize DPPH radicals and neutralize their reactivity may be enhanced at larger concentrations by giving electrons or hydrogen atoms. In addition, ZnONPs exhibit a rise in DPPH scavenging activity as their concentration increases. This suggests that ZnONPs have antioxidant qualities, most likely because they can interact with DPPH radicals by surface reactions or by releasing ions that can contribute hydrogen or electrons. The varied ways in which ZnONPs and plant extract interact with and neutralise free radicals are important to comprehend since it provides information on how these compounds may be used as antioxidants in the future.
6 Conclusion
This research marks a significant advancement in nanotechnology, particularly in the green synthesis of ZnONPs using G. ulmifolia leaf extract. The synthesized ZnONPs, characterized by their spherical shape and size of 19 to 41 nm, underscore the efficacy of plant extracts in producing nanoparticles with controlled morphologies and dimensions. The optimization of synthesis parameters achieving an ideal pH of 10 and a temperature of 60 °C highlights the critical role of environmental conditions in tailoring nanoparticle properties for specific applications.
The ZnONPs synthesized in this study demonstrate remarkable antioxidant and antibacterial activities, suggesting their potential as effective agents in biomedical fields. Their capability to scavenge free radicals and inhibit microbial growth positions them as promising candidates for applications in disease prevention, treatment, and medical imaging. The use of G. ulmifolia leaf extract not only provides a sustainable and eco-friendly approach to nanoparticle synthesis but also adds value to this method by leveraging the intrinsic properties of the plant materials.
Looking forward, the integration of ZnONPs into various chemical reactions and formulations could revolutionize therapeutic strategies, particularly in targeting cancerous cells, combating microbial infections, and enhancing diagnostic imaging techniques. The potential for these nanoparticles to be incorporated into drug delivery systems, wound healing materials, and antimicrobial coatings opens new avenues for research and development in nanomedicine.
Further in vivo studies are imperative to ascertain the safety, efficacy, and biocompatibility of ZnONPs, particularly in clinical settings. The findings from this research will pave the way for practical implementations that could significantly impact public health and environmental sustainability.
Data availability
Data and materials are included in this review.
References
Zhou XQ, Hayat Z, Zhang DD, Li MY, Hu S, Wu Q, Cao YF, Yuan Y (2023) Zinc Oxide nanoparticles: synthesis, characterization, modification, and applications in food and agriculture. Processes 11(4):1193
Jiang J, Pi J, Cai J (2018) The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem 2018:1062562
Rahman A, Harunsani MH, Tan AL, Ahmad N, Min BK, Khan MM (2021) Influence of Mg and Cu dual-doping on phytogenic synthesized ZnO for light induced antibacterial and radical scavenging activities. Mater Sci Semicond Process 128:105761
Rahman A, Tan AL, Harunsani MH, Ahmad N, Hojamberdiev M, Khan MM (2021) Visible light induced antibacterial and antioxidant studies of ZnO and Cu-doped ZnO fabricated using aqueous leaf extract of Ziziphus mauritiana Lam. J Environ Chem Eng 9(4):105481
Kołodziejczak-Radzimska A, Jesionowski T (2014) Zinc oxide—from synthesis to application: a review. Materials 7(4):2833–2881
Rahman A, Harunsani MH, Tan AL, Ahmad N, Khan MM (2021) Antioxidant and antibacterial studies of phytogenic fabricated ZnO using aqueous leaf extract of Ziziphus mauritiana Lam. Chem Pap 75:3295–3308
Pushpalatha C, Suresh J, Gayathri VS, Sowmya SV, Augustine D, Alamoudi A, Zidane B, Mohammad Albar NH, Patil S (2022) Zinc oxide nanoparticles: a review on its applications in dentistry. Front Bioeng Biotechnol 10:917990
Rahman A, Harunsani MH, Tan AL, Ahmad N, Hojamberdiev M, Khan MM (2021) Effect of Mg doping on ZnO fabricated using aqueous leaf extract of Ziziphus mauritiana Lam for antioxidant and antibacterial studies. Bioprocess Biosyst Eng 44:875–889
Ahmad M, Rehman W, Khan MM, Qureshi MT, Gul A, Haq S, Ullah R, Rab A, Menaa F (2021) Phytogenic fabrication of ZnO and gold decorated ZnONPs for photocatalytic degradation of Rhodamine B. J Environ Chem Eng 9(1):104725
Khan MM, Harunsani MH, Tan AL, Hojamberdiev M, Poi YA, Ahmad N (2020) Antibacterial studies of ZnO and Cu-doped ZnONPs synthesized using aqueous leaf extract of Stachytarpheta jamaicensis. Bionanoscience 10:1037–1048
Mahamuni PP, Patil PM, Dhanavade MJ, Badiger MV, Shadija PG, Lokhande AC, Bohara RA (2019) Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem Biophys Rep 17:71–80
Barzinjy AA, Hamad SM, Abdulrahman AF, Biro SJ, Ghafor AA (2020) Biosynthesis, characterization and mechanism of formation of ZnONPs using Petroselinum crispum leaf extract. Curr Org Synth 17(7):558–566
Bandeira M, Giovanela M, Roesch-Ely M, Devine DM, da Silva CJ (2020) Green synthesis of zinc oxide nanoparticles: a review of the synthesis methodology and mechanism of formation. Sustain Chem Pharm 15:100223
Mahmoud AE (2020) Eco-friendly reduction of graphene oxide via agricultural byproducts or aquatic macrophytes. Mater Chem Phys 253:123336
Mahmoud AE, Stolle A, Stelter M (2018) Sustainable synthesis of high-surface-area graphite oxide via dry ball milling. ACS Sustain Chem Eng 6(5):6358–6369
Mellinas C, Jiménez A, Garrigós MD (2019) Microwave-assisted green synthesis and antioxidant activity of selenium nanoparticles using Theobroma cacao L. bean shell extract. Molecules 24(22):4048
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci 9(1):1–7
Ebadi M, Zolfaghari MR, Aghaei SS, Zargar M, Shafiei M, Zahiri HS, Noghabi KA (2019) A bio-inspired strategy for the synthesis of zinc oxide nanoparticles (ZnO NPs) using the cell extract of cyanobacterium Nostoc sp. EA03: from biological function to toxicity evaluation. RSC Adv 9(41):23508–23525
Mahmoud AE, Fawzy M (2021) Nanosensors and nanobiosensors for monitoring the environmental pollutants. In: Waste recycling technologies for nanomaterials manufacturing, pp 229–46
Mousavi SM, Hashemi SA, Ghasemi Y, Atapour A, Amani AM, Savar Dashtaki A, Babapoor A, Arjmand O (2018) Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif Cells Nanomed Biotechnol 46(sup3):855–872
Hussain I, Singh NB, Singh A, Singh H, Singh SC (2016) Green synthesis of nanoparticles and its potential application. Biotechnol Lett 38:545–560
Singh P, Kim YJ, Zhang D, Yang DC (2016) Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol 34(7):588–599
Nilavukkarasi M, Vijayakumar S, Prathipkumar S (2020) Capparis zeylanica mediated bio-synthesized ZnONPs as antimicrobial, photocatalytic and anti-cancer applications. Mater Sci for Energy Technol 3:335–343
Hussain A, Oves M, Alajmi MF, Hussain I, Amir S, Ahmed J, Rehman MT, El-Seedi HR, Ali I (2019) Biogenesis of ZnONPs using Pandanus odorifer leaf extract: anticancer and antimicrobial activities. RSC Adv 9(27):15357–15369
Isa EM, Shameli K, Jusoh NC, Sukri SM, Ismail NA (2021) Variation of green synthesis techniques in fabrication of zinc oxide nanoparticles–a mini review. Mater Sci Eng 1051(1):012079
Loganathan S, Shivakumar MS, Karthi S, Nathan SS, Selvam K (2021) Metal oxide nanoparticle synthesis (ZnO-NPs) of Knoxia sumatrensis (Retz.) DC. Aqueous leaf extract and It’s evaluation of their antioxidant, anti-proliferative and larvicidal activities. Toxicol Rep 8:64–72
Mahmoud AE, El-Maghrabi N, Hosny M, Fawzy M (2022) Biogenic synthesis of reduced graphene oxide from Ziziphus spina-christi (Christ’s thorn jujube) extracts for catalytic, antimicrobial, and antioxidant potentialities. Environ Sci Pollut Res 29(59):89772–89787
Iqbal N, Iqubal SS, Khan AA, Mohammed T, Alshabi AM, Aazam ES, Rafiquee MZA (2021) Effect of CTABr (surfactant) on the kinetics of formation of silver nanoparticles by Amla extract. J Mol Liq 329:115537. https://doi.org/10.1016/j.molliq.2021.115537
Becker J, Manske C, Randl S (2022) Green chemistry and sustainability metrics in the pharmaceutical manufacturing sector. Curr Opin Green Sustain Chem 33:100562
Rajasekharreddy P, Rani PU, Sreedhar B (2010) Qualitative assessment of silver and gold nanoparticle synthesis in various plants: a photobiological approach. J Nanoparticle Res 12:1711–1721
Dolcet P, Casarin M, Maccato C, Bovo L, Ischia G, Gialanella S, Mancin F, Tondello E, Gross S (2012) Miniemulsions as chemical nanoreactors for the room temperature synthesis of inorganic crystalline nanostructures: ZnO colloids. J Mater Chem 22(4):1620–1626
Medina-Flores D, Ulloa-Urizar G, Camere-Colarossi R, Caballero-GarcÝa S, Mayta-Tovalino F, del Valle-Mendoza J (2016) Antibacterial activity of Bixa orellana L. (achiote) against Streptococcus mutans and Streptococcus sanguinis. Asian Pac J Trop Biomed 6(5):400–403
Rahman FU, Khan S, Rahman MU, Zaib R, Rahman MU, Ullah R, Zahoor M, Kamran AW (2024) Effect of ionic strength on DNA–dye interactions of Victoria blue B and methylene green using UV–visible spectroscopy. Z Phys Chem 238(1):173–186
Nazir S, Zhang JM, Junaid M, Saleem S, Ali A, Ullah A, Khan S (2024) Metal-based nanoparticles: basics, types, fabrications and their electronic applications. Z Phys Chem. https://doi.org/10.1515/zpch-2023-0375
Carofiglio M, Barui S, Cauda V, Laurenti M (2020) Doped zinc oxide nanoparticles: synthesis, characterization and potential use in nanomedicine. Appl Sci 10(15):5194
Al Awadh AA, Shet AR, Patil LR, Shaikh IA, Alshahrani MM, Nadaf R, Mahnashi MH, Desai SV, Muddapur UM, Achappa S, Hombalimath VS, Khan AA, Gouse HSM, Iqubal SMS, Kumbar V (2022) Sustainable synthesis and characterization of zinc oxide nanoparticles using Raphanus sativus extract and its biomedical applications. Crystals 12(8):1142. https://doi.org/10.3390/cryst12081142
Fardood ST, Ramazani A, Moradi S, Asiabi PA (2017) Green synthesis of zinc oxide nanoparticles using Arabic gum and photocatalytic degradation of direct blue 129 dye under visible light. J Mater Sci Mater Electron 28:13596–13601. https://doi.org/10.1007/s10854-017-7199-5
Vinayagam R, Selvaraj R, Arivalagan P, Varadavenkatesan T (2020) Synthesis, characterization and photocatalytic dye degradation capability of Calliandra haematocephala-mediated zinc oxide nanoflowers. J Photochem Photobiol B Biol 203:111760. https://doi.org/10.1016/j.jphotobiol.2019.111760
Khan S, Ullah I, Khan H, Rahman FU, Rahman MU, Saleem MA, Nazir S, Ali A, Ullah A (2024) Green synthesis of AgNPs from leaves extract of Saliva Sclarea, their characterization, antibacterial activity, and catalytic reduction ability. Z Phys Chem. https://doi.org/10.1515/zpch-2023-0363
Nannapaneni UR, Rayapati SR (2019) A novel approach towards green synthesis of zinc nano particles from Guazuma ulmifolia (Lam) for their anti oxidant activity study. Indian Drugs 56(2):16–20. https://doi.org/10.53879/id.56.02.11587
Gupta M, Tomar RS, Kaushik S, Mishra RK, Sharma D (2018) Effective antimicrobial activity of green ZnO nano particles of Catharanthus roseus. Front microbiol 9:2030
Shaikh IA, Turakani B, Mahnashi MH, Alqahtani AS, Hariri SH, Ghoneim MM, Ebrahim HA, El-Sherbiny M, Mannasaheb BA, Muddapur UM, Khuwaja G, Khan AA, Dafalla SE, Begum T, Iqubal SMS (2023) Environmentally friendly production, characterization, and evaluation of ZnO NPs from Bixa orellana leaf extract and assessment of its antimicrobial activity. J King Saud Univ Sci 35(11):102957. https://doi.org/10.1016/j.jksus.2023.102957
Muddapur UM, Turakani B, Jalal NA, Ashgar SS, Momenah AM, Alshehri OM, Mahnashi MH, Shaikh IA, Khan AA, Dafalla SE, Malpani J, Manjunath S, Begum S, Khuwja G, Iqubal SMS (2023) Phytochemical screening of Bixa orellana and preliminary antidiabetic, antibacterial, antifibrinolytic, anthelmintic, antioxidant, and cytotoxic activity against lung cancer (A549) cell lines. J King Saud Univ Sci 35(5):102683. https://doi.org/10.1016/j.jksus.2023.102683
El-Bassossy TA, Abdelgawad AA, Abo-Zaid MA, Amin AH, El-Agamy SA, Elazab KM, Ismail AH (2023) Evaluation of the immunomodulatory, antioxidant, and histopathological effects of Cymbopogon schoenanthus essential oil extract on kidney and spleen in BALB/C Mice. J Umm Al-Qura Univ Appll Sci 5:1–2
Jha V, Kadam P, Jain T, Bhargava A, Marick A, Saiya B, Maiti S, Pandya S, Patel R, Jadhav N (2023) Investigation of physico-chemical properties and evaluation of the biological potential of essential oil extracted from Artemisia pallens. J Umm Al-Qura Univ Appll Sci 30:1–4
Khan AU, Malik N, Singh B, Ansari NH, Rehman M, Yadav A (2023) Biosynthesis, and characterization of Zinc oxide nanoparticles (ZnONPs) obtained from the extract of waste of strawberry. J Umm Al-Qura Univ Appll Sci 20:1–8
Vera J, Herrera W, Hermosilla E, Díaz M, Parada J, Seabra AB, Tortella G, Pesenti H, Ciudad G, Rubilar O (2023) Antioxidant activity as an indicator of the efficiency of plant extract-mediated synthesis of zinc oxide nanoparticles. Antioxidants 12(4):784. https://doi.org/10.3390/antiox12040784
Acknowledgements
The authors are thankful to Ibn Sina National College for Medical Studies, Jeddah, Kingdom of Saudi Arabia and KLE Technological University, BVB, Hubballi, Karnataka, India.
Funding
There are no sources of financial funding and support.
Author information
Authors and Affiliations
Contributions
Conceptualization, Salah Eldeen Dafalla, Uday M. Muddapur, Shivani Angadi, Laxmikant R. Patil; Data curation, Nayef Abdulaziz Aldabaan, Uday M. Muddapur; Formal analysis, Uday M. Muddapur, Shivani Angadi, Laxmikant R. Patil, Aejaz Abdullatif Khan, S.M. Shakeel Iqubal; Methodology, Ibrahim Ahmed Shaikh, Aejaz Abdullatif Khan, S. M. Shakeel Iqubal, Anil R. Shet, Shivalingsarj V. Desai, Veeranna S. Hombalimath; Writing—original draft, Shivani Angadi, Laxmikant R. Patil, Salah Eldeen Dafalla Writing—review & editing, Ibrahim Ahmed Shaikh, Aejaz Abdullatif Khan, S. M. Shakeel Iqubal, Anil R. Shet, Shivalingsarj V. Desai, Veeranna S. Hombalimath.
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dafalla, S.E., Aldabaan, N.A., Muddapur, U.M. et al. Green and sustainable synthesis of the ZnONPs using leaf extract of Guazuma ulmifolia for antioxidant and antimicrobial activities. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00161-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-024-00161-x