Abstract
Cadmium is the most harmful soil pollutant due to its long biological half-life. In the present study, the effect of Cd on ammonium assimilation in Arabidopsis thaliana wild-type Col0 was investigated. Thirty-day-old Arabidopsis seedlings were exposed to 20 µM CdCl2 during different exposure times (0, 1, 2, 3, and 7 days). Seedling growth decreased under Cd stress mainly after 7 days of Cd exposure. Cd stress caused a gradual decrease in soluble leaf protein and induced an increase in leaf ammonium and the content of free amino acids such as glutamine (Gln), glutamate (Glu), asparagine (Asn), and proline (Pro), which may be related to the increase in protease activity. The results showed that the activities of Glutamine Synthetase (GS) and glutamate dehydrogenase (GDH) were inversely related. Cd stress led to an increase in GDH activity, whereas GS activity decreased. GDH activity on polyacrylamide gels showed that Cd induced both β- and α-enriched isoforms. Therefore, this study confirms that the ammonium assimilation process plays an important role for plants in adapting to Cd stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A member of the mustard family, Arabidopsis thaliana is a little flowering plant [1]. Its generation time is 4–5 weeks and it is studied as plant model in plant physiology. Cadmium contamination of agricultural lands is largely due to the application of Cd-containing fertilizers and sewage sludge [2]. Cd is considered one of the most highly toxic pollutants to all living organisms [3, 4]. Several studies have demonstrated its cytotoxicity on plant growth and physiology [3, 5, 6]. Cd stress in plants leads to various stress symptoms such as chlorosis, disturbances in mineral nutrition, nitrogen and carbohydrate metabolism and a reduction in biomass production [6, 7]. The photosynthetic apparatus is particularly vulnerable to Cd, and a reduction in photosynthesis is a common response in plants exposed to Cd [8].
Cd decreased plant growth, photosynthetic rate and nitrogen metabolism [9,10,11]. Cd can also alter plant mineral uptake through its effects on the availability of minerals from the soil or by reducing the population of soil microbes [12]. Several hyperaccumulating plant species have been used for soil Cd decontamination as tobacco plants [9, 13].
A growing number of studies address the effects of Cd stress on photosynthetic CO2 fixation [14, 15]. Although photosynthetic CO2 fixation plays an important role in plant yield, ammonium assimilation contributes greatly to plant production. However, the effects of Cd stress on ammonium assimilation have been poorly studied. Therefore, in the present study, we attempted to explain the Cd-induced temporal changes in ammonium assimilation in Arabidopsis thaliana. Ammonium assimilation by metabolism of ammonium into amino acids and amides is part of the process of ammonium detoxification in plants [16]. Glutamate dehydrogenase (GDH) and glutamine synthetase (GS) are among the enzymes involved in ammonium assimilation. GDH activity is thought to occur in mitochondria, whereas GS activity occurs in chloroplasts [16]. Therefore, the present study investigated the Cd-induced temporal changes in the activity of these two key enzymes involved in ammonium assimilation, GDH and GS.
2 Materials and methods
2.1 Plant material and growth conditions
Arabidopsis thaliana wild-type Col0, used for the experiments, were obtained from research lab of “Adaptation des Plantes à leur Environnement”, INRA France. Seeds were sterilized and stratified at 4 °C for 4 days. Plants were grown under hydroponic culture in a growth chamber with an 8-h-light/16-h-dark cycle and 80% relative humidity. The plants were supplied with the basic nutrient medium containing 8 mM KNO3, 1 mM MgSO4, 1 mM KH2PO4, 2 mM CaNO3, 5 µM MnSO4, 30 µM H3BO3, 1 µM ZnSO4, 1 µM CuSO4, and 30 µM K- iron-EDTA. Plants were grown for 4 weeks and were then divided into two groups. The first was maintained on the same media (control). The second was transferred to basic nutrient medium containing 20 μM of CdCl2 solution. The treatments were applied for different times (1 day, 2 days, 3 days and 7 days).
2.2 Metabolites measurements
2.2.1 Ammonium content
Ammonium was extracted from the leaves at 4 °C with 0.3 mM H2SO4 and 0.5% (w/v) polyclar AT. The ammonium content was quantified according to the reaction of Berthelot modified by Weatherburn [17].
2.2.2 Protein content
Soluble protein contents in both lines and all treatments were quantified using Coomassie Brilliant Blue with bovine serum albumin as a protein standard [18].
2.2.3 Protease activity
Protease activity was measured using the method of Weckenmann and Martin [19], using azocasein as substrate. Absorbance of the released-azo-dye was measured at 340 nm and one unit of activity was defined as the activity producing an increase of 0.01 unit of absorbance during the 1 h incubation.
2.2.4 Metabolite's measurement
Total amino acid content was assayed by the method of Rosen [20] using glutamine as a reference. Individual amino acid composition was determined using ion exchange chromatography [21].
2.3 Enzyme assays
2.3.1 Glutamine synthetase
The enzyme essay was carried out on each line and each treatment. Frozen samples were homogenized in a cold mortar and pestle with grinding medium containing 25 mM Tris–HCl buffer (pH 7.6), 1 mM MgCl2, 1 mM EDTA, 14 mM 2-mercaptoethanol and 1% (w/v) polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 25,000g for 30 min at 4 °C (centrifuge refrigerator Eppendorf 5810r). GS activity was determined using hydroxylamine as a substrate, and the formation of γ-glutamylhydroxamate (γ-GHM) was quantified with acidified ferric chloride [22].
2.3.2 Glutamate dehydrogenase
The enzyme essay was carried on each line and on each treatment. GDH extractions were performed according to the method described by Magalhaes and Huber [23]. Frozen samples were homogenized in a cold mortar and pestle with 100 mM Tris–HCl (pH 7.5), 14 mM 2-mercaptoethanol, and 1% (w/v) PVP. The extract was centrifuged at 12,000g for 15 min at 4 °C. GDH activity was determined by following the absorbance changes at 340 nm.
2.3.3 Detection of GDH activity on the gel
Leaf soluble proteins were extracted from frozen material in cold extraction buffer containing 100 mM Tricine, 1 mM EDTA, 40 mM CaCl2, 0.5% (w/v) PVP, 0.1% (v/v) 2-mercaptoethanol, and 1 mM AEBSF (4-(2-aminoethyl)-benzenesulfonyl fluoride). The protein separation was carried out in a 1 mm-thick non-denaturing gel, as described previously. Equal amounts of protein (60 µg) were loaded into each gel track. Native PAGE of the partially purified GDH extracts was performed by the method of Davis [24] on 5% running gel with a 4% stacking gel. The buffer system was 100 mM Tris–Glycine adjusted to pH 8 with HCl. Running was at 4 °C, 120 V, for about 2 h, and bands containing GDH activity were visualized with the tetrazolium system. The staining solution contained 150 mM Tris–HCl (pH 8.8), 50 mM glutamate, 0.5 mM NAD+, 0.5 mM NBT (Nitro Blue Tetrazolium chloride), and phenazine.
2.4 Statistical analysis
The data presented in the figures is the average of at least six replicates per exposure treatment and means ± confidence limits at alpha = 0.05 level. Two-way analysis of variance (ANOVA) and Tukey’s HSD tests was used to determine significant differences between means treatments at probability level ≤ 0.05, using the SPSS software 20.1 (Version IBM. 20.0. 2004).
3 Results
3.1 Plant growth
Cd treatment decreased seedling growth in Arabidopsis thaliana, mainly after 7 days of the treatment (Fig. 1). The growth response of Arabidopsis thaliana plants to 20 µM CdCl2 during different exposure time (0, 1, 2, 3, and 7 days) is shown in Fig. 2. Since the first day of Cd exposure, seedling fresh weight (FW) decreased by about 35% compared to control (Fig. 2). The FW reduction became less important after 2 and 3 days, and it didn’t exceed 13% compared to the control. The effect of Cd treatment was more pronounced at the 7 day of Cd exposure, and FW decreased more to reach more than 49%.
Cadmium effect on FW (FW: mg) in Arabidopsis thaliana after different Cd exposure time (0, 1, 2, 3, and 7 days). The data presented are the means values of six replicates, ± standard error. Lowercase letters (a, b) compare control and treated samples at the same Cd exposure time. Bars denoted by same letters do not significantly differ by the Tukey test at 5% probability
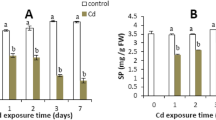
3.2 Soluble protein content
The results showed that the leaf soluble protein (SP) content decreased (about 15%) since the first day of Cd treatment compared to the control. After 7 days of Cd treatment the leaf soluble protein decreased more than 30% compared to the control (Fig. 3).
Cadmium effect on leaf soluble protein (SP: mg/g FW) in Arabidopsis thaliana after different Cd exposure time (0, 1, 2, 3, and 7 days). The data presented are the means values of six replicates, ± standard error. Lowercase letters (a, b) compare control and treated samples at the same Cd exposure time. Bars denoted by same letters do not significantly differ by the Tukey test at 5% probability
3.3 Ammonium content
The presence of Cd in the growth media induced an endogenous ammonium accumulation in leaves of about 25% since the first day of treatment, compared with the control and a gradual increase was associated with the increasing time of exposure. This increase was more pronounced in the 7 day of Cd addition, the endogenous ammonium content increased by 50%, in comparison with the control (Fig. 4).
Cadmium effect on ammonium content (μmol/gFW) in leaves of Arabidopsis thaliana after different Cd exposure time (0, 1, 2, 3, and 7 days). The data presented are the means values of six replicates, ± standard error. Lowercase letters (a, b) compare control and treated samples at the same Cd exposure time. Bars denoted by same letters do not significantly differ by the Tukey test at 5% probability
3.4 Protease activity
The Cd treatment induced an increase of protease activity since the first day of treatment. A remarkable increase in the protease activity was noticed After 2 days of Cd treatment and the percentage of the increase was about 40% compared to control. After 7 days of treatment, the protease activity increased by only 30% refers to control (Fig. 5).
Cadmium effect on protease activity (units/gFW/h) in leaves of Arabidopsis thaliana after different Cd exposure time (0, 1, 2, 3, and 7 days). Data are means of six replicates ± CL at 0.05 levels. Lowercase letters (a, b) compare control and treated samples at the same Cd exposure time. Bars denoted by same letters do not significantly differ by the Tukey test at 5% probability
3.5 Cd effects on ammonium-assimilating enzymes
3.5.1 Glutamine synthetase activity
Results showed that after only 1 day of Cd exposure, GS activity decreased. The GS activity decreased progressively with time of Cd exposure. After 3 days, the decrease of GS activity remained important (20%) referring to the control. While, the Cd treatment for 7 days induced a small increase of GS activity by about 4% refer to control (Fig. 6).
Cadmium effect on Glutamine synthetase activity (nmol GHM/ mg SP/min) in leaves of Arabidopsis thaliana after different Cd exposure time (0, 1, 2, 3, and 7 days). Data are means of six replicates ± CL at 0.05 levels. Lowercase letters (a, b) compare control and treated samples at the same Cd exposure time. Bars denoted by same letters do not significantly differ by the Tukey test at 5% probability
3.5.2 Glutamate dehydrogenase activity and GDH subunit patterns
As shown in Fig. 7A, the Cd presence in growth media caused a significant stimulation of GDH dehydrogenase activity. This increase happened especially after 7 days of exposure. The GDH activity rise of about 13%, 20% and 50% respectively after 2, 3 and 7 days of treatment.
Cadmium effect on A glutamate dehydrogenase activity (µmol NADH oxidized /mg SP/h) in leaves of Arabidopsis thaliana after different Cd exposure time (0, 1, 2, 3, and 7 days). Data are means of six replicates ± CL at 0.05 levels. Lowercase letters (a, b) compare control and treated samples at the same Cd exposure time. Bars denoted by same letters do not significantly differ by the Tukey test at 5% probability. B The amount of each GDH protein was expressed as % relative to the corresponding protein amount prior to Cd treatment
GDH activity on a polyacrylamide gel revealed the presence of seven GDH isoform patterns formed by the binding of six of the two α and β subunits (Fig. 7B).
The upper GDH band was formed by six β subunits and the base band by six α subunits.
Detection of GDH activity on gel and quantification of proteins showed higher staining intensity, especially in his fourth band. Results showed that Cd induced both α and β subunits. GDH activity increased gradually until his 7 days of Cd exposure, and the band density of the fourth isoform was more than twice as dense compared to controls. GDH activity on polyacrylamide gels was consistent with GDH measured in Arabidopsis leaves after different Cd exposure times under Cd treatment. Also in this experiment, the overall increase in NADH-GDH activity paralleled the decrease in GS activity.
3.6 Free amnio acid level
During the experimental period, total amino acid levels remained unchanged in the leaves after 1 day of Cd exposure (Fig. 8A). In the stressed plants, amino acid levels increased visibly after 2 days of Cd treatment by about 17% referring to control (Fig. 8A). After 2 days of Cd exposure, a slight increase in Glu (Glutamate) and Asn (Asparagine) levels was detected in leaves (Fig. 8B). A small Cd inhibitory effect was revealed on Gln level (Fig. 8B). In contrast, Proline (Pro) level was increased by about 23% referring to control in treated leaves.
Cadmium effect on A free amino acid level (nmol/µl) in leaves of Arabidopsis thaliana after different Cd exposure time (0, 1, 2, and 3 days). Data are means of six replicates ± CL at 0.05 levels. Means sharing at least one letter are not significantly different according to the Tukey test at P ≤ 0.05. B The amount of each amino acid (GLN, Glu, ASN, proline) after 2 days of cadmium exposure in leaves of Arabidopsis thaliana. The results are the mean of three replicates. Lowercase letters (a, b) compare control and treated samples at the same Cd exposure time. Bars denoted by same letters do not significantly differ by Tukey’s test at 5% probability
4 Discussion
The incorporation of nitrogen into the formation of various cellular components, such as proteins and nucleic acids, constitutes a significant mechanism through which nitrogen metabolism facilitates plant growth. It can be inferred that any circumstance, including both biotic and abiotic stresses, which cause a decrease in the availability of nitrogen, particularly through ammonium absorption, will have an adverse impact on the overall growth and productivity of plants. Several studies explain the close relationship between nitrogen supply and cadmium. In 2020, Yang et al. demonstrated that nitrogen supply increases Cd uptake and accumulation in plants [25]. On the other hand, Vazquez et al. explained that ammonium, as the only source of nitrogen, decreases Cd accumulation and allows Cd retention in roots [26].
In fact, it has been found that the presence of Cd in plants has a negative impact on their productivity and has a tendency to accumulate in various plant organs, which can have a detrimental effect on vital physiological processes [27]. The exposure of plants to cadmium is recognized to result in a decrease in measurable growth parameters such as height, root length, fresh weight (FW), and dry weight (DW). The magnitude of deleterious effects exhibited significant variation in relation to the particular plant species, genotype, and plant tissue. Several sensitive growth parameters have been utilized as indicators of phytotoxicity induced by Cd [28, 29].
The toxicity of Cd in plants is known to vary based on several factors such as growing conditions, exposure time, exposure length, and plant age, as documented by previous literature [30]. Several plant species, comprising tobacco, Pisum sativum, and chickpea, have been associated with cadmium toxicity [9, 29,30,31]. A plausible explanation for the observed decline in protein concentration [32] could be a deceleration in protein synthesis or acceleration in protein degradation.
The ammonium ions hold significant importance in the nitrogen metabolism of plants, as cited in several studies [16]. The formation of ammonium arises as a consequence of nitrogen assimilation through nitrate reduction, deamination of amino acids, and photorespiration, as reported by a previous study [16]. Previous studies have reported analogous elevations in the accumulation of endogenous ammonium with Cd exposure in seedlings of tomato and rice [33, 34]. The stimulation of protease activity was observed under cadmium-induced stress conditions in various plant species, such as tobacco [9], tomato seedlings [34], and barley [35]. The elevation observed in the concentration of ammonium in foliage can plausibly be attributed to heightened protease activity (refer to Fig. 4). GS enzyme serves as the principal catalyst accountable for the process of ammonium assimilation in plants [16]. The introduction of Cd resulted in a significant reduction in the overall activity of GS, which had a prominent involvement in the process of ammonium assimilation [36, 37]. It is evident that the accretion of ammonium in leaves is consequent upon the attenuation in the functionality of the enzyme glutamine synthetase. Although the function of GDH in higher plants remains controversial, the current findings indicate a negative correlation between the enzymatic activities of GS and GDH. The concurrent decline in GS activity and augmentation in GDH activity may plausibly be accounted for by this phenomenon. Therefore, the elevation in GDH activity may facilitate the assimilation of accumulated ammonia subsequent to the decline in GS activity. Henceforth, the observed rise in GDH activity may potentially serve as a compensatory mechanism to counterbalance the reduction in GS activity. This finding is in accordance with prior studies conducted on tomato seedlings that have undergone treatment with Cd [34].
The aminating activity of GDH is believed to be linked to the process of ammonium detoxification under Cd stress [34]. The present investigation's results provide corroboration for the postulation that the glutamine synthetase/glutamate synthase (GS/GOGAT) pathway, responsible for ammonium assimilation, may undergo a change towards the GDH pathway when subjected to Cd treatment [35].
Exposure to Cd led to a decrease in Gln level could be due to a decrease in GS activity.
On the other hand, as depicted in Fig. 8B, Cd induced an increase in glutamate (Glu) and asparagine (Asn) levels in leaves, the increase in Glu and Asn content was reported by Zhu, et al., in C. crepidioides plants [38]. The greater accumulation of free amino acids, especially Gln and Asn, might be related to Cd tolerance [38].
Proline acts as a heavy metal chelator, thereby alleviating heavy metal stress [39, 40]. Cd treatment can lead to an increase in proline content in many plant species and appears to correspond with Cd-induced changes in water balance. Pandey and Sharma [41] demonstrated that Cd induces an increase in Proline concentration in cabbage leaves and suggested a relationship with the altered water status of treated plants. Free Proline chelates Cd ions and forms a non-toxic Cd-proline complex [42]. Proline is also involved in antioxidant protection. Matysik et al. investigated the role of proline in plant protection against reactive oxygen species damage [43]. Proline as osmoprotectant, biochar [44], Acetate [45] and nitrogen salts [46] were different solutions proposed to reduce Cd toxicity in plants.
5 Conclusion
The plant growth and ammonium assimilation appear to be affected by Cd. In the current study, we investigated the link between the exposure period in Arabidopsis thaliana seedlings under Cd stress and the two essential enzymes involved in ammonium assimilation, GS and GDH. Increased GDH activity may compensate for decreased GS activity caused by Cd exposure. In Arabidopsis thaliana, the accumulation of free amino acids, particularly Gln, Asn, and Proline, may be related to cadmium resistance.
Data availability
My manuscript has no associated data.
References
Leslie SL, Barbara RH-E, Elliot MM (1984) The DNA of Arabidopsis thaliana. Mol Gen Genet 194:15–23. https://doi.org/10.1007/BF00383491
McLaughlin MJ, Singh BR (1999) Cadmium in soils and plants. Springer, Berlin, pp 1–9
Li Y, Rahman SU, Qiu Z, Shahzad SM, Nawaz MF, Huang J, Naveed S, Li L, Wang X, Cheng H (2023) Toxic effects of cadmium on the physiological and biochemical attributes of plants, and phytoremediation strategies: a review. Environ Pollut 325:121433. https://doi.org/10.1016/j.envpol.2023.121433
Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, Wenjun M, Farooq M (2021) Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf 211:111887. https://doi.org/10.1016/j.ecoenv.2020.111887
Shanshan L, Meng W, Zhongqiu Z, Xiaoyue L, Yun H, Shibao C (2018) Alleviation of cadmium phytotoxicity to wheat is associated with Cd re-distribution in soil aggregates as affected by amendments. RSC Adv 8:17426–17434. https://doi.org/10.1039/C8RĂ6A
Santos C, Monteiro M, Dias MC (2010) Cadmium toxicity in crops. A review (Environmental science, engineering and technology). Nova Science Publishers Incorporated, New York
Azevedo H, Glória Pinto CG, Fernandes J, Loureiro S, Santos C (2005) Cadmium effects on sunflower growth and photosynthesis. J Plant Nutr 28:2211–2220. https://doi.org/10.1080/01904160500324782
Burzyński M, Kłobus G (2004) Changes of photosynthetic parameters in cucumber leaves under Cu, Cd, and Pb stress. Photosynthetica 42:505–510. https://doi.org/10.1007/S11099-005-0005-2
Maaroufi Dguimi H, Alshehri K, Zaghdoud C, Albaggar AK, Debouba M (2019) Effect of cadmium repartition on nitrogen metabolism in tobacco seedlings. Open Access Libr J 6:e4000. https://doi.org/10.4236/oalib.1104000
Wahid A, Ghani A, Ali I, Ashraf MY (2007) Effects of cadmium on carbon and nitrogen assimilation in shoots of mungbean [Vigna radiata (L.) Wilczek] seedlings. J Agron Crop Sci 193:357–365. https://doi.org/10.1111/j.1439-037X.2007.00270.x
Wang L, Zhou Q, Ding L, Sun Y (2008) Effect of cadmium toxicity on nitrogen metabolism in leaves of Solanum nigrum L. as a newly found cadmium hyperaccumulator. J Hazard Mater 154:818–825. https://doi.org/10.1016/j.jhazmat.2007.10.097
Moreno JL, Hernández T, Garcia C (1999) Effects of a cadmium-contaminated sewage sludge compost on dynamics of organic matter and microbial activity in an arid soil. Biol Fertil Soils 28:230–237. https://doi.org/10.1007/s003740050487
Wang J, Lu X, Zhang J, Ouyang Y, Wei G, Xiong Y (2020) Rice intercropping with alligator flag (Thalia dealbata): a novel model to produce safe cereal grains while remediating cadmium contaminated paddy soil. J Hazard Mater 394:122505. https://doi.org/10.1016/j.jhazmat.2020.122505
Jing D, Fei-bo WU, Guo-ping Z (2005) Effect of cadmium on growth and photosynthesis of tomato seedlings. J Zhejiang Univ Sci B 6:974. https://doi.org/10.1631/jzus.2005.B0974
Wang H, Zhao SC, Liu RL, Zhou W, Jin JY (2009) Changes of photosynthetic activities of maize (Zea mays L.) seedlings in response to cadmium stress. Photosynthetica 47:277–283. https://doi.org/10.1007/s11099-009-0043-2
Miflin BJ, Lea PJ (1976) The pathway of nitrogen assimilation in plants. Phytochemistry 15:873–885. https://doi.org/10.1016/S0031-9422(00)84362-9
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Ann Chem 39(8):971–974. https://doi.org/10.1021/ac60252a045
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Weckenmann D, Martin P (1984) Endopeptidase activity and nitrogen mobilization in senescing leaves of Nicotiana rustica in light and dark. Physiol Plant 60:333–340. https://doi.org/10.1111/j.1399-3054.1984.tb06072.x
Rosen H (1957) A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys 67:10–15. https://doi.org/10.1016/0003-9861(57)90241-2
Suzuki A, Vergnet C, Morot-Gaudry J, Zehnacker C, Grosclaude J (1994) Immunological characterization of ferrodoxin and methyl viologen interacting domains of glutamate synthase using monoclonal antibodies. Plant Physiol Biochem 32:619–626
Wallsgrove RM, Lea PJ, Miflin BJ (1979) Distribution of the enzymes of nitrogen assimilation within the pea leaf cell. Plant Physiol 63:232–236. https://doi.org/10.1104/pp.63.2.232
Magalhaes JR, Huber DM (1991) Free ammonia, free amino acids, and enzyme activity in maize tissue treated with methionine sulfoximine. J Plant Nutr 14:883–895. https://doi.org/10.1080/01904169109364249
Davis BJ (1964) Disc electrophoresis II. Method and application to human serum proteins. Ann N Y Acad Sci 121:404–427. https://doi.org/10.1111/j.1749-6632.1964.tb14213.x
Yang Y, Xiong J, Tao L, Cao Z, Tang W, Zhang J, Yu X, Fu G, Zhang X, Lu Y (2020) Regulatory mechanisms of nitrogen (N) on cadmium (Cd) uptake and accumulation in plants: a review. Sci Total Environ 708:135186. https://doi.org/10.1016/j.scitotenv.2019.135186
Vazquez A, Recalde L, Cabrera A, Groppa MD, Benavides MP (2020) Does nitrogen source influence cadmium distribution in Arabidopsis plants? Ecotoxicol Environ Saf 191:110163
Gill SS, Khan NA, Tuteja N (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulphur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 182:112–120. https://doi.org/10.1016/j.plantsci.2011.04.018
Faizan S, Kausar S, Perveen R (2011) Varietal differences for cadmium-induced seedling mortality, foliar toxicity symptoms, plant growth, proline and nitrate reductase activity in chickpea (Cicer arietinum L.). Biol Med 3:196–206
Mondal NK, Chittaranjan D, Satinath R, Datta JK, Arnab B (2013) Effect of varying cadmium stress on chickpea (Cicer arietinum L.) seedlings: an ultrastructural study. Ann Environ Sci 7: 59–70. http://hdl.handle.net/2047/d20018674
Metwally A, Safronova VI, Belimov AA, Dietz K (2005) Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J Exp Bot 56:167–178. https://doi.org/10.1093/jxb/eri017
Bovet L, Rossi L, Lugon-Moulin N (2006) Cadmium partitioning and gene expression studies in Nicotiana tabacum and Nicotiana rustica. Physiol Plant 128:466–475. https://doi.org/10.1111/j.1399-3054.2006.00756.x
Balestrasse KB, Benavides MP, Gallego SM, Tomaro ML (2003) Effect of cadmium stress on nitrogen metabolism in nodules and roots of soybean plants. Funct Plant Biol 30:57–64. https://doi.org/10.1071/FP02074
Chien H, Kao CH (2000) Accumulation of ammonium in rice leaves in response to excess cadmium. Plant Sci 156:111–115. https://doi.org/10.1016/s0168-9452(00)00234-x
Nasraoui HA, Maaroufi Dguimi H, Bouthour D, Gouia H, ChaffeiHaouari C (2012) Adjustments of nitrogen and carbon metabolisms were the mainly strategy followed in ammonium-fed tomato to alleviate cadmium toxicity. Int J Curr Res 4:258–263
Boussama N, Ouariti O, Ghorbal MH (1999) Changes in growth and nitrogen assimilation in barley seedlings under cadmium stress. J Plant Nutr 22:731–752. https://doi.org/10.1080/01904169909365668
Tobin AK, Yamaya T (2001) Cellular compartmentation of ammonium assimilation in rice and barley. J Exp Bot 52(356):591–604. https://doi.org/10.1093/jexbot/52.356.591
Miflin Ben J, HabashDimah Z (2002) The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot 53(370):979–987. https://doi.org/10.1093/jexbot/53.370.979
Zhu G, Xiao H, Guo Q, Zhang Z, Zhao J, Yang D (2018) Effects of cadmium stress on growth and amino acid metabolism in two Compositae plants. Ecotoxicol Environ Saf 158:300–308. https://doi.org/10.1016/j.ecoenv.2018.04.045
Chia MA, Lombardi AT, da Graça Gama Melão M, Parrish CC (2015) Combined nitrogen limitation and cadmium stress stimulate total carbohydrates, lipids, protein and amino acid accumulation in Chlorella vulgaris (Trebouxiophyceae). Aquat Toxicol 160:87–95. https://doi.org/10.1016/j.aquatox.2015.01.002
Nikolić N, Kojić D, Pilipović A, Pajević S, Krstić B, Borišev M, Orlović S (2008) Responses of hybrid poplar to cadmium stress: photosynthetic characteristics, cadmium and proline accumulation, and antioxidant enzyme activity. Acta Biol Cracov Bot 50:95–103
Pandey N, Sharma CP (2002) Effect of heavy metals Co2, Ni2 and Cd2 on growth and metabolism of cabbage. Plant Sci 163:753–758
Sharma SS, Schat H, Vooijs R (1998) In vitro alleviation of heavy metal-induced enzyme inhibition by proline. Phytochemistry 49:1531–1535. https://doi.org/10.1016/s0031-9422(98)00282-9
Matysik J, Alia Bhalu B, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532. https://www.jstor.org/stable/24105959
Ghassemi-Golezani K, Farhangi-Abriz S (2023) Biochar related treatments improved physiological performance, growth and productivity of Mentha crispa L. plants under fluoride and cadmium toxicities. Ind Crops Prod 194:116287
Hossain MS, Abdelrahman M, Tran CD, Nguyen KH, Chu HD, Watanabe Y, Fujita M, Tran LSP (2022) Modulation of osmoprotection and antioxidant defense by exogenously applied acetate enhances cadmium stress tolerance in lentil seedlings. Environ Pollut 308:119687. https://doi.org/10.1016/j.envpol.2022.119687
Paul S, Guha T, Dey S, Paul S, Kundu R (2022) Amelioration of cadmium toxicity by enhancing nitrogen assimilation and photosynthetic activity by two different nitrogen supplements in rice (Oryza sativa L.) cv. Lalat. Plant Stress 4:100082
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by HMD and FOA. The first draft of the manuscript was written by HMD. FOA revised manuscript and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dguimi, H.M., Alzahrani, F.O. Changes in growth, ammonium assimilation and amino acid levels in leaves of Arabidopsis thaliana under cadmium treatment. J.Umm Al-Qura Univ. Appll. Sci. 9, 317–324 (2023). https://doi.org/10.1007/s43994-023-00048-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00048-3